Combination of Transcriptomic and Proteomic Approaches Helps to Unravel the Protein Composition of Chelidonium Majus L
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ATP-Citrate Lyase Has an Essential Role in Cytosolic Acetyl-Coa Production in Arabidopsis Beth Leann Fatland Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2002 ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis Beth LeAnn Fatland Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Molecular Biology Commons, and the Plant Sciences Commons Recommended Citation Fatland, Beth LeAnn, "ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis " (2002). Retrospective Theses and Dissertations. 1218. https://lib.dr.iastate.edu/rtd/1218 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. ATP-citrate lyase has an essential role in cytosolic acetyl-CoA production in Arabidopsis by Beth LeAnn Fatland A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Physiology Program of Study Committee: Eve Syrkin Wurtele (Major Professor) James Colbert Harry Homer Basil Nikolau Martin Spalding Iowa State University Ames, Iowa 2002 UMI Number: 3158393 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -
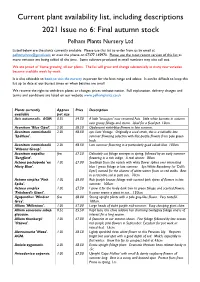
Current Plant Availability List, Including Descriptions 2021 Issue No 6: Final Autumn Stock Pelham Plants Nursery Ltd
Current plant availability list, including descriptions 2021 Issue no 6: Final autumn stock Pelham Plants Nursery Ltd Listed below are the plants currently available. Please use this list to order from us by email at [email protected] or over the phone on 07377 145970. Please use the most recent version of this list as more varieties are being added all the time. Some cultivars produced in small numbers may also sell out. We are proud of ‘home growing’ all our plants. The list will grow and change substantially as many new varieties become available week by week. It is also advisable to book to visit the nursery in person for the best range and advice. It can be difficult to keep this list up to date at our busiest times or when batches are small. We reserve the right to withdraw plants or changes prices without notice. Full explanation, delivery charges and terms and conditions are listed on our website www.pelhamplants.co.uk Plants currently Approx Price Description available pot size Acis autumnalis. AGM. 0.5L £4.50 A little 'Leucojum' now renamed Acis. Little white bonnets in autumn over grassy foliage and stems. Ideal for a focal pot. 10cm. Aconitum 'Blue Opal'. 2.0L £8.50 Opalescent violet-blue flowers in late summer. Aconitum carmichaelii 2.0L £8.50 syn. Late Vintage. Originally a seed strain, this is a valuable late 'Spätlese'. summer flowering selection with lilac-purple flowers from pale green buds. Aconitum carmichaelii 2.0L £8.50 Late summer flowering in a particularly good cobalt blue. -

Isolation and Identification of Alkaloids from Macleaya Microcarpa by UHPLC–Q-TOF-MS and Their Cytotoxic Activity in Vitro, An
Sai et al. BMC Chemistry (2020) 14:5 https://doi.org/10.1186/s13065-020-0660-1 BMC Chemistry RESEARCH ARTICLE Open Access Isolation and identifcation of alkaloids from Macleaya microcarpa by UHPLC– Q-TOF-MS and their cytotoxic activity in vitro, antiangiogenic activity in vivo Chunmei Sai1,2, Jian’an Wang1, Binjie Li3, Lin Ding1, Huiyun Wang1, Qibao Wang1, Huiming Hua3, Fangpeng Zhang2 and Qiang Ren1* Abstract Background: Extensive bioactivities of alkaloids from the genus Macleaya (Macleaya cordata (Willd.) R. Br. and Macleaya microcarpa (Maxim.) Fedde) have been widely reported, as well as more and more concerned from the sci- entifc communities. However, systematic research on the phytochemical information of M. microcarpa is incomplete. The aim of this study was to rapidly and conveniently qualitative analyze alkaloids from M. microcarpa by ultra-perfor- mance liquid chromatography/quadrupole-time-of-fght mass spectrometry (UHPLC–Q-TOF-MS) using accurate mass weight and characteristic fragment ions, furthermore separate and identify the main alkaloids, test antitumor activity in vitro and antiangiogenic activity in vivo. Results: A total of 14 alkaloids from fruits of M. microcarpa were identifed by UHPLC–Q-TOF-MS, including 5 pro- topines, 2 benzophenanthridines, 1 dimer, 1 dihydrobenzophenanthridines and 5 unknown structure compounds. Two major alkaloids were isolated by various column chromatographic methods. Their structures were determined by NMR data and related literatures. The two major alkaloids were evaluated for intro cytotoxic activities against HL-60, MCF-7, A-549, and in vivo antiangiogenic activity using transgenic zebrafsh. Conclusions: Current qualitative method based on UHPLC–Q-TOF-MS technique provided a scientifc basis for isola- tion, structural identifcation, and in vitro or in vivo pharmacological further study of alkaloids from M. -

Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders
REVIEW published: 21 August 2018 doi: 10.3389/fphar.2018.00557 Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders Maria A. Neag 1, Andrei Mocan 2*, Javier Echeverría 3, Raluca M. Pop 1, Corina I. Bocsan 1, Gianina Cri¸san 2 and Anca D. Buzoianu 1 1 Department of Pharmacology, Toxicology and Clinical Pharmacology, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, 2 Department of Pharmaceutical Botany, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, 3 Department of Environmental Sciences, Universidad de Santiago de Chile, Santiago de Chile, Chile Edited by: Berberine-containing plants have been traditionally used in different parts of the world for Anna Karolina Kiss, the treatment of inflammatory disorders, skin diseases, wound healing, reducing fevers, Medical University of Warsaw, Poland affections of eyes, treatment of tumors, digestive and respiratory diseases, and microbial Reviewed by: Pinarosa Avato, pathologies. The physico-chemical properties of berberine contribute to the high diversity Università degli Studi di Bari Aldo of extraction and detection methods. Considering its particularities this review describes Moro, Italy various methods mentioned in the literature so far with reference to the most important Sylwia Zielinska, Wroclaw Medical University, Poland factors influencing berberine extraction. Further, the common separation and detection *Correspondence: methods like thin layer chromatography, high performance liquid chromatography, and Andrei Mocan mass spectrometry are discussed in order to give a complex overview of the existing [email protected] methods. Additionally, many clinical and experimental studies suggest that berberine Specialty section: has several pharmacological properties, such as immunomodulatory, antioxidative, This article was submitted to cardioprotective, hepatoprotective, and renoprotective effects. -

Highly Variable Chloroplast Genome from Two Endangered Papaveraceae Lithophytes Corydalis Saxicola and C
Highly variable chloroplast genome from two endangered Papaveraceae lithophytes Corydalis saxicola and C. tomentella fengming Ren Chinese Academy of Medical Sciences & Peking Union Medical College Institute of Medicinal Plant Development Liqiang Wang Heze University Ying Li Chinese Academy of Medical Sciences & Peking Union Medical College Institute of Medicinal Plant Development Wei Zhuo Chongqing Insititute of Medicinal Plant Cultivation Zhichao Xu Chinese Academy of Medical Sciences & Peking Union Medical College Institute of Medicinal Plant Development Haojie Guo Wuhu Institute of Technology Yan Liu Chongqing Institute of Medicinal Plant Cultivation Ranran Gao Chinese Academy of Medical Sciences & Peking Union Medical College Institute of Medicinal Plant Development Jingyuan Song ( [email protected] ) Research article Keywords: Corydalis saxicola, Corydalis tomentella, Papaveraceae, taxonomic study, cp genome Posted Date: April 17th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-18411/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Ecology and Evolution on March 19th, 2021. See the published version at https://doi.org/10.1002/ece3.7312. Page 1/22 Abstract Backgroud: Corydalis DC., the largest genus of Papaveraceae, is recognized as one of the most taxonomically challenging plant taxa. However, no complete chloroplast (cp) genome for this genus has been reported to date. Results: We sequenced four complete cp genomes of two anities Corydalis saxicola and C. tomentellav of the genus Corydalis, compared these cp genomes with each other and others from Papaveraceae, and analyzed the phylogenetic relationships based on the sequences of common CDS. -

Bocconia and Macleaya Author(S): J
Bocconia and Macleaya Author(s): J. Hutchinson Source: Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew), Vol. 1920, No. 8 (1920), pp. 275-282 Published by: Springer on behalf of Royal Botanic Gardens, Kew Stable URL: http://www.jstor.org/stable/4120232 Accessed: 14-06-2016 14:47 UTC Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at http://about.jstor.org/terms JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Royal Botanic Gardens, Kew, Springer are collaborating with JSTOR to digitize, preserve and extend access to Bulletin of Miscellaneous Information (Royal Botanic Gardens, Kew) This content downloaded from 131.172.36.29 on Tue, 14 Jun 2016 14:47:17 UTC All use subject to http://about.jstor.org/terms 275 an old but no longer common species, 28 ft. high and 5 ft. in girth of trunk; and a fine healthy tree of the newer Japanese M. hypoleuca over 30 ft. high. There is a good collection of hardy bamboos on the lower, damper part of the garden, and it was interesting to see Phyllo- stachys aurea flowering, although not gratifying, since it portends the flowering and consequent death of the species throughout the country. XLIV.-BOCCONIA AND MACLEAYA. J. HUTCHINSON. The genus Bocconia (Papaveraceae) was founded by Linnaeus* in 1737, the type species being B. -

University of Groningen Exploring the Cofactor-Binding and Biocatalytic
University of Groningen Exploring the cofactor-binding and biocatalytic properties of flavin-containing enzymes Kopacz, Malgorzata IMPORTANT NOTE: You are advised to consult the publisher's version (publisher's PDF) if you wish to cite from it. Please check the document version below. Document Version Publisher's PDF, also known as Version of record Publication date: 2014 Link to publication in University of Groningen/UMCG research database Citation for published version (APA): Kopacz, M. (2014). Exploring the cofactor-binding and biocatalytic properties of flavin-containing enzymes. Copyright Other than for strictly personal use, it is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), unless the work is under an open content license (like Creative Commons). The publication may also be distributed here under the terms of Article 25fa of the Dutch Copyright Act, indicated by the “Taverne” license. More information can be found on the University of Groningen website: https://www.rug.nl/library/open-access/self-archiving-pure/taverne- amendment. Take-down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Downloaded from the University of Groningen/UMCG research database (Pure): http://www.rug.nl/research/portal. For technical reasons the number of authors shown on this cover page is limited to 10 maximum. Download date: 29-09-2021 Exploring the cofactor-binding and biocatalytic properties of flavin-containing enzymes Małgorzata M. Kopacz The research described in this thesis was carried out in the research group Molecular Enzymology of the Groningen Biomolecular Sciences and Biotechnology Institute (GBB), according to the requirements of the Graduate School of Science, Faculty of Mathematics and Natural Sciences. -

Discovery of Industrially Relevant Oxidoreductases
DISCOVERY OF INDUSTRIALLY RELEVANT OXIDOREDUCTASES Thesis Submitted for the Degree of Master of Science by Kezia Rajan, B.Sc. Supervised by Dr. Ciaran Fagan School of Biotechnology Dublin City University Ireland Dr. Andrew Dowd MBio Monaghan Ireland January 2020 Declaration I hereby certify that this material, which I now submit for assessment on the programme of study leading to the award of Master of Science, is entirely my own work, and that I have exercised reasonable care to ensure that the work is original, and does not to the best of my knowledge breach any law of copyright, and has not been taken from the work of others save and to the extent that such work has been cited and acknowledged within the text of my work. Signed: ID No.: 17212904 Kezia Rajan Date: 03rd January 2020 Acknowledgements I would like to thank the following: God, for sending me angels in the form of wonderful human beings over the last two years to help me with any- and everything related to my project. Dr. Ciaran Fagan and Dr. Andrew Dowd, for guiding me and always going out of their way to help me. Thank you for your patience, your advice, and thank you for constantly believing in me. I feel extremely privileged to have gotten an opportunity to work alongside both of you. Everything I’ve learnt and the passion for research that this project has sparked in me, I owe it all to you both. Although I know that words will never be enough to express my gratitude, I still want to say a huge thank you from the bottom of my heart. -

The Ins and Outs of Vanillyl Alcohol Oxidase: Identification of Ligand Migration Paths
RESEARCH ARTICLE The ins and outs of vanillyl alcohol oxidase: Identification of ligand migration paths Gudrun Gygli1, Maria FaÂtima Lucas2, Victor Guallar2,3, Willem J. H. van Berkel1* 1 Laboratory of Biochemistry, Wageningen University & Research, Stippeneng 4, WE Wageningen, The Netherlands, 2 Joint BSC-IRB Research Program in Computational Biology, Barcelona Supercomputing Center, Jordi Girona 29, Barcelona, Spain, 3 Institucio Catalana de Recerca i Estudis AvancËats (ICREA), Passeig LluõÂs Companys 23, Barcelona, Spain * [email protected] a1111111111 a1111111111 a1111111111 Abstract a1111111111 a1111111111 Vanillyl alcohol oxidase (VAO) is a homo-octameric flavoenzyme belonging to the VAO/ PCMH family. Each VAO subunit consists of two domains, the FAD-binding and the cap domain. VAO catalyses, among other reactions, the two-step conversion of p-creosol (2- methoxy-4-methylphenol) to vanillin (4-hydroxy-3-methoxybenzaldehyde). To elucidate how different ligands enter and exit the secluded active site, Monte Carlo based simulations OPEN ACCESS have been performed. One entry/exit path via the subunit interface and two additional exit Citation: Gygli G, Lucas MF, Guallar V, van Berkel paths have been identified for phenolic ligands, all leading to the si side of FAD. We argue WJH (2017) The ins and outs of vanillyl alcohol oxidase: Identification of ligand migration paths. that the entry/exit path is the most probable route for these ligands. A fourth path leading to PLoS Comput Biol 13(10): e1005787. https://doi. the re side of FAD has been found for the co-ligands dioxygen and hydrogen peroxide. org/10.1371/journal.pcbi.1005787 Based on binding energies and on the behaviour of ligands in these four paths, we propose Editor: Donald Jacobs, UNC Charlotte, UNITED a sequence of events for ligand and co-ligand migration during catalysis. -

The Central Role of Acetyl-Coa in Plant Metabolism, As Examined Through Studies of ATP Citrate Lyase and the Bio1 Mutant of Arabidopsis Elizabeth K
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 2004 The central role of acetyl-CoA in plant metabolism, as examined through studies of ATP citrate lyase and the bio1 mutant of Arabidopsis Elizabeth K. Winters Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Genetics Commons, Molecular Biology Commons, and the Plant Sciences Commons Recommended Citation Winters, Elizabeth K., "The ec ntral role of acetyl-CoA in plant metabolism, as examined through studies of ATP citrate lyase and the bio1 mutant of Arabidopsis " (2004). Retrospective Theses and Dissertations. 1203. https://lib.dr.iastate.edu/rtd/1203 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. The central role of acetyl-CoA in plant metabolism, as examined through studies of ATP citrate lyase and the biol mutant of Arabidopsis by Elizabeth K. Winters A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Plant Physiology Program of Study Committee: Eve Syrkin Wurtele, Co-major Professor Basil J. Nikolau, Co-major Professor Thomas Baum James T. Colbert David J. Oliver Mark Westgate Iowa State University Ames, Iowa 2004 UMI Number: 3158378 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. -

Supporting Information
Supporting Information Vert et al. 10.1073/pnas.0803996105 Fig. S1. ARF2 interacts with BIN2 but not BES1 or BZR1. Shown is a representative example of staining for lacZ reporter expression for a variety of plasmid combinations. Staining is not detected for any of the plasmids on their own. Yeast transformed with BIN2 and ARF2 are the only tested strains to show detectable reporter activity. A–D show increasing strength of interactions for well characterized control proteins. Vert et al. www.pnas.org/cgi/content/short/0803996105 1of28 Fig. S2. ARF2 protein accumulation is not altered by BR treatment. Western blot using anti-ARF2 antibodies on total extracts from plants without or with 1 M BL. The nonspecific band indicated with an asterisk (Lower) serves as a loading control. Vert et al. www.pnas.org/cgi/content/short/0803996105 2of28 Fig. S3. BIN2 can phosphorylate and reduce DNA binding of BES1. (A) Our preparation of BIN2 kinase can phosphorylate BES1. Upper shows Coomassie blue staining, and Lower shows Western blot using anti-MPB antibody. (B) In vitro DNA binding assays show that phosphorylation of BES1 by BIN2 greatly reduces BES1 binding to DNA, consistent with the results reported by Vert and Chory (1). Equivalent amounts of protein were incubated with DNA-coated beads. Proteins bound to the DNA were then pulled down, released by boiling, and run on an SDS/PAGE gel. Western blots using indicated antibodies are shown. Very little to no DNA is recovered when BIN2 is added to the reaction in the absence of BES1. 1. Vert G, Chory J (2006) Downstream nuclear events in brassinosteroid signalling. -

Supplemental Table S1: Comparison of the Deleted Genes in the Genome-Reduced Strains
Supplemental Table S1: Comparison of the deleted genes in the genome-reduced strains Legend 1 Locus tag according to the reference genome sequence of B. subtilis 168 (NC_000964) Genes highlighted in blue have been deleted from the respective strains Genes highlighted in green have been inserted into the indicated strain, they are present in all following strains Regions highlighted in red could not be deleted as a unit Regions highlighted in orange were not deleted in the genome-reduced strains since their deletion resulted in severe growth defects Gene BSU_number 1 Function ∆6 IIG-Bs27-47-24 PG10 PS38 dnaA BSU00010 replication initiation protein dnaN BSU00020 DNA polymerase III (beta subunit), beta clamp yaaA BSU00030 unknown recF BSU00040 repair, recombination remB BSU00050 involved in the activation of biofilm matrix biosynthetic operons gyrB BSU00060 DNA-Gyrase (subunit B) gyrA BSU00070 DNA-Gyrase (subunit A) rrnO-16S- trnO-Ala- trnO-Ile- rrnO-23S- rrnO-5S yaaC BSU00080 unknown guaB BSU00090 IMP dehydrogenase dacA BSU00100 penicillin-binding protein 5*, D-alanyl-D-alanine carboxypeptidase pdxS BSU00110 pyridoxal-5'-phosphate synthase (synthase domain) pdxT BSU00120 pyridoxal-5'-phosphate synthase (glutaminase domain) serS BSU00130 seryl-tRNA-synthetase trnSL-Ser1 dck BSU00140 deoxyadenosin/deoxycytidine kinase dgk BSU00150 deoxyguanosine kinase yaaH BSU00160 general stress protein, survival of ethanol stress, SafA-dependent spore coat yaaI BSU00170 general stress protein, similar to isochorismatase yaaJ BSU00180 tRNA specific adenosine