Refrigeration Systems for Achieving Cryogenic Temperatures Ronald G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stirling Refrigerator
Stirling Refrigerator Mid-Point Report Luis Gardetto Ahmad Althomali Abdulrahman Alazemi Faiez Alazmi John Wiley 2017-2018 Project Sponsor: Dr. David Trevas Faculty Advisor: Mr. David Willy Instructor: Dr. Sarah Oman DISCLAIMER This report was prepared by students as part of a university course requirement. While considerable effort has been put into the project, it is not the work of licensed engineers and has not undergone the extensive verification that is common in the profession. The information, data, conclusions, and content of this report should not be relied on or utilized without thorough, independent testing and verification. University faculty members may have been associated with this project as advisors, sponsors, or course instructors, but as such they are not responsible for the accuracy of results or conclusions. i Executive Summary Refrigeration is a ubiquitous application of engineering concepts, with implications like food preservation techniques, superconducting-magnet involving techniques and crystal harvesting techniques. The refrigeration process involves the removal of excess heat from specified objects, thereby enhancing the cooling or the chilling effect. Refrigerators come in many sizes and shapes, according to the available expertise, materials, and applications. With the advancement of technology and the emergence of various contemporary issues such as cost, size, and dangerous chemicals among others, researchers are embarking on studies, which can facilitate the design and development of a more efficient refrigerator, capable of performing the desired work as presupposed with minimal problems. The current proposal focuses on Stirling cryocoolers, which have been recognized as one of the most efficient coolers of the current era. The key advantages of these chillers include low power consumption rates as well as the use of non-hazardous refrigerants. -

An Analysis of the Brayton Cycle As a Cryogenic Refrigerator
jbrary, E-01 Admin. BJdg. OCT 7 1968 NBS 366 An Analysis of the Brayton Cycle As a Cryogenic Refrigerator ITATIONAL BURSAL' OF iTANDABD8 LIBRABT MAR 6 J973 V* 0F c J* 0a <5 Q \ v S. DEPARTMENT OF COMMERCE in Q National Bureau of Standards %. *^CAU 0? * : NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maxi- mum application of the physical and engineering sciences to the advancement of tech- nology in industry and commerce. To this end the Bureau conducts research and provides central national services in three broad program areas and provides cen- tral national services in a fourth. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, and the Center for Radiation Research. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement, coor- dinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. The Institute consists of an Office of Standard Reference Data and a group of divisions organized by the following areas of science and engineering Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Phys- 2 2 ics—Cryogenics-—Radio Physics-—Radio Engineering —Astrophysics —Time and Frequency. -

Fuel Cells Versus Heat Engines: a Perspective of Thermodynamic and Production
Fuel Cells Versus Heat Engines: A Perspective of Thermodynamic and Production Efficiencies Introduction: Fuel Cells are being developed as a powering method which may be able to provide clean and efficient energy conversion from chemicals to work. An analysis of their real efficiencies and productivity vis. a vis. combustion engines is made in this report. The most common mode of transportation currently used is gasoline or diesel engine powered automobiles. These engines are broadly described as internal combustion engines, in that they develop mechanical work by the burning of fossil fuel derivatives and harnessing the resultant energy by allowing the hot combustion product gases to expand against a cylinder. This arrangement allows for the fuel heat release and the expansion work to be performed in the same location. This is in contrast to external combustion engines, in which the fuel heat release is performed separately from the gas expansion that allows for mechanical work generation (an example of such an engine is steam power, where fuel is used to heat a boiler, and the steam then drives a piston). The internal combustion engine has proven to be an affordable and effective means of generating mechanical work from a fuel. However, because the majority of these engines are powered by a hydrocarbon fossil fuel, there has been recent concern both about the continued availability of fossil fuels and the environmental effects caused by the combustion of these fuels. There has been much recent publicity regarding an alternate means of generating work; the hydrogen fuel cell. These fuel cells produce electric potential work through the electrochemical reaction of hydrogen and oxygen, with the reaction product being water. -

A Turbo-Brayton Cryocooler for Future European Observation Satellite Generation
C19_078 1 A Turbo-Brayton Cryocooler for Future European Observation Satellite Generation J. Tanchon1, J. Lacapere1, A. Molyneaux2, M. Harris2, S. Hill2, S.M. Abu-Sharkh2, T. Tirolien3 1Absolut System SAS, Seyssinet-Pariset, France 2Ofttech, Gloucester, United Kingdom 3European Space Agency, Noordwijk, The Netherlands ABSTRACT Several types of active cryocoolers have been developed for space and military applications in the last ten years. Performances and reliability continuously increase to follow the requirements evolution of new generations of satellite: less power consumption, more cooling capacity, increased life duration. However, in addition to this increase of performances and reliability, the microvibrations re- quirement becomes critical. In fact, with the development of vibration-free technologies, classical Earth Observation cryocoolers (Stirling, Pulse Tube) will become the main source of microvibra- tions on-board the satellite. A new generation cryocooler is being developed at Absolut System using very high speed turbomachines in order to avoid any generated perturbations below 1000 Hz. This development is performed in the frame of ESA Technical Research Program - 4000113495/15/NL/KML. This paper presents the status of this development project based on Reverse Brayton cycle with very high speed turbomachines. INTRODUCTION The space cryogenics sector is characterized by a large number of applications (detection, im- aging, sample conservation, propulsion, telecommunications etc.) that lead to various requirements (temperature range, microvibration, lifetime, power consumption etc.) that can be met by different solutions (Stirling or JT coolers, mechanical or sorption compressors etc.). The recent years saw, in Europe, the developments of coolers to meet Earth Observation mission UHTXLUHPHQWVWKDWDUHFDSDEOHWRSURYLGHVLJQL¿FDQWFRROLQJSRZHUDWDQRSHUDWLRQDOWHPSHUDWXUH around 50K (for IR detection). -

First and Second Law Evaluation of Combined Brayton-Organic Rankine Power Cycle
Journal of Thermal Engineering, Vol. 6, No. 4, pp. 577-591, July, 2020 Yildiz Technical University Press, Istanbul, Turkey FIRST AND SECOND LAW EVALUATION OF COMBINED BRAYTON-ORGANIC RANKINE POWER CYCLE Önder Kaşka1, Onur Bor2, Nehir Tokgöz3* Muhammed Murat Aksoy4 ABSTRACT In the present work, we have conducted thermodynamic analysis of an organic Rankine cycle (ORC) using waste heat from intercooler and regenerator in Brayton cycle with intercooling, reheating, and regeneration (BCIRR). First of all, the first law analysis is used in this combined cycle. Several outputs are revealed in this study such as the cycle efficiencies in Brayton cycle which is dependent on turbine inlet temperature, intercooler pressure ratios, and pinch point temperature difference. For all cycles, produced net power is increased because of increasing turbine inlet temperature. Since heat input to the cycles takes place at high temperatures, the produced net power is increased because of increasing turbine inlet temperature for all cycles. The thermal efficiency of combined cycle is higher about 11.7% than thermal efficiency of Brayton cycle alone. Moreover, the net power produced by ORC has contributed nearly 28650 kW. The percentage losses of exergy for pump, turbine, condenser, preheater I, preheater II, and evaporator are 0.33%, 33%, 22%, 23%, 6%, and16% respectively. The differences of pinch point temperature on ORC net power and efficiencies of ORC are investigated. In addition, exergy efficiencies of components with respect to intercooling pressure ratio and evaporator effectiveness is presented. Exergy destructions are calculated for all the components in ORC. Keywords: Brayton Cycle, Organic Rankine Cycle, Bottoming Cycle, Pinch Point Temperature, Waste Heat, Energy and Exergy Analysis INTRODUCTION Thermal energy systems as well as corresponding all parts have been challenged to improve overall efficiency due to lack of conventional fuels, reduce climate change and so on for recent years. -

Design of a Cryogenic Turbine for a Hybrid Cryocooler
Design of a Cryogenic Turbine for a Hybrid Cryocooler by Thomas L. Fraser A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science (Mechanical Engineering) at the UNIVERISTY OF WISCONSIN-MADISON 2006 Approved by _____________________________________________ ______________ Professor Gregory F. Nellis Date i Abstract The hybrid pulse tube-reverse Brayton cycle cryocooler has the potential for cooling to temperatures on the order of 10 K. By using the rectifying interface which converts the oscillating pulse tube flow to continuous flow, both vibrations and low temperature regenerator losses are overcome, making the hybrid an ideal candidate for cooling infrared focal plane arrays which demand low temperature and low vibration. However, the turboexpander within the reverse Brayton cycle is complex and its performance is highly dependent on the performance of its subcomponents, thus necessitating a model predicting the turboexpander performance. A model was developed to predict the performance of the reverse Brayton cycle stage including the recuperative heat exchanger and turboexpander components. The turboexpander was numerically modeled in detail to include the sub-models of rotordynamics, the thermal and leakage performance of the seal, and the turboalternator. Where possible, the models were verified against either an analytical model or experimental data. A parametric analysis was carried out to determine the optimal design and conditions for the turboexpander. ii Acknowledgements First and foremost I would like to thank Greg Nellis. Besides being my advisor for this project he is also responsible for sparking an interest in energy science through his heat transfer courses I took as an undergrad. -

Measuring the Steady State Thermal Efficiency of a Heating System
Measuring the Steady State Thermal Efficiency of a Heating System. Last week you had a tour of “Colby Power and Light” and had a chance to see Gus Libby’s really cool, campus-scale, multi-million dollar calorimeter. This week we will use lab-scale calorimeters to measure the heat and carbon content of different fuels (methane, propane, butane, and gasoline). Our simple calorimeters were designed by Whitney King and Chuck Jones, Science Division Instrument Wizard, to simulate the highly efficient HTP Versa Flame fire-tube boiler. The lab has three parts: 1) Calibrate the calorimeter 2) Measure the heat content of different fuels 3) Compare the measured heat content to the calculated heat content 1) Calibrate the Calorimeter. You lab instructor will demonstrate the assembly of the laboratory- scale fire-tube calorimeter. The heat transferred from the burning fuel to the calorimeter can be determined from the mass of the object being heated, the heat capacity of the object and the change in temperature. � = ���� ∗ �� ∗ ∆� We will begin the experiment by adding a known amount of heat to our calorimeters from a coffee cup heater. The heater is a simple resistor that converts electrical energy into heat. The heater has a resistance of 47.5 ohms and is plugged into a 120 V outlet. Recalling your electricity basics, we know that V=IR where V is the voltage in Figure 1. HTP Versa Flame Fire Tube volts, I is the current in amps, and R is the Boiler. Brown Tubes carry the hot resistance in ohms. The power, P, consumed by combustion gasses inside of the the heater is volts times current, water tank where they condense releasing the maximum available 2 heat. -

Supercritical Carbon Dioxide(S-CO2) Power Cycle for Waste Heat Recovery: a Review from Thermodynamic Perspective
processes Review Supercritical Carbon Dioxide(s-CO2) Power Cycle for Waste Heat Recovery: A Review from Thermodynamic Perspective Liuchen Liu, Qiguo Yang and Guomin Cui * School of Energy and Power Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China; [email protected] (L.L.); [email protected] (Q.Y.) * Correspondence: [email protected] Received: 11 October 2020; Accepted: 13 November 2020; Published: 15 November 2020 Abstract: Supercritical CO2 power cycles have been deeply investigated in recent years. However, their potential in waste heat recovery is still largely unexplored. This paper presents a critical review of engineering background, technical challenges, and current advances of the s-CO2 cycle for waste heat recovery. Firstly, common barriers for the further promotion of waste heat recovery technology are discussed. Afterwards, the technical advantages of the s-CO2 cycle in solving the abovementioned problems are outlined by comparing several state-of-the-art thermodynamic cycles. On this basis, current research results in this field are reviewed for three main applications, namely the fuel cell, internal combustion engine, and gas turbine. For low temperature applications, the transcritical CO2 cycles can compete with other existing technologies, while supercritical CO2 cycles are more attractive for medium- and high temperature sources to replace steam Rankine cycles. Moreover, simple and regenerative configurations are more suitable for transcritical cycles, whereas various complex configurations have advantages for medium- and high temperature heat sources to form cogeneration system. Finally, from the viewpoints of in-depth research and engineering applications, several future development directions are put forward. This review hopes to promote the development of s-CO2 cycles for waste heat recovery. -

25 Kw Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications
25 kW Low-Temperature Stirling Engine for Heat Recovery, Solar, and Biomass Applications Lee SMITHa, Brian NUELa, Samuel P WEAVERa,*, Stefan BERKOWERa, Samuel C WEAVERb, Bill GROSSc aCool Energy, Inc, 5541 Central Avenue, Boulder CO 80301 bProton Power, Inc, 487 Sam Rayburn Parkway, Lenoir City TN 37771 cIdealab, 130 W. Union St, Pasadena CA 91103 *Corresponding author: [email protected] Keywords: Stirling engine, waste heat recovery, concentrating solar power, biomass power generation, low-temperature power generation, distributed generation ABSTRACT This paper covers the design, performance optimization, build, and test of a 25 kW Stirling engine that has demonstrated > 60% of the Carnot limit for thermal to electrical conversion efficiency at test conditions of 329 °C hot side temperature and 19 °C rejection temperature. These results were enabled by an engine design and construction that has minimal pressure drop in the gas flow path, thermal conduction losses that are limited by design, and which employs a novel rotary drive mechanism. Features of this engine design include high-surface- area heat exchangers, nitrogen as the working fluid, a single-acting alpha configuration, and a design target for operation between 150 °C and 400 °C. 1 1. INTRODUCTION Since 2006, Cool Energy, Inc. (CEI) has designed, fabricated, and tested five generations of low-temperature (150 °C to 400 °C) Stirling engines that drive internally integrated electric alternators. The fifth generation of engine built by Cool Energy is rated at 25 kW of electrical power output, and is trade-named the ThermoHeart® Engine. Sources of low-to-medium temperature thermal energy, such as internal combustion engine exhaust, industrial waste heat, flared gas, and small-scale solar heat, have relatively few methods available for conversion into more valuable electrical energy, and the thermal energy is usually wasted. -

Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle
entropy Article Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle Fuli Zhu 1,2,3, Lingen Chen 1,2,3,* and Wenhua Wang 1,2,3 1 Institute of Thermal Science and Power Engineering, Naval University of Engineering, Wuhan 430033, China; [email protected] (F.Z.); [email protected] (W.W.) 2 Military Key Laboratory for Naval Ship Power Engineering, Naval University of Engineering, Wuhan 430033, China 3 College of Power Engineering, Naval University of Engineering, Wuhan 430033, China * Correspondence: [email protected] or [email protected]; Tel.: +86-27-8361-5046; Fax: +86-27-8363-8709 Received: 20 January 2018; Accepted: 2 March 2018; Published: 5 March 2018 Abstract: An irreversible Maisotsenko reciprocating Brayton cycle (MRBC) model is established using the finite time thermodynamic (FTT) theory and taking the heat transfer loss (HTL), piston friction loss (PFL), and internal irreversible losses (IILs) into consideration in this paper. A calculation flowchart of the power output (P) and efficiency (η) of the cycle is provided, and the effects of the mass flow rate (MFR) of the injection of water to the cycle and some other design parameters on the performance of cycle are analyzed by detailed numerical examples. Furthermore, the superiority of irreversible MRBC is verified as the cycle and is compared with the traditional irreversible reciprocating Brayton cycle (RBC). The results can provide certain theoretical guiding significance for the optimal design of practical Maisotsenko reciprocating gas turbine plants. Keywords: finite-time thermodynamics; irreversible Maisotsenko reciprocating Brayton cycle; power output; efficiency 1. Introduction The revolutionary Maisotsenko cycle (M-cycle), utilizing the psychrometric renewable energy from the latent heat of water evaporating, was firstly provided by Maisotsenko in 1976. -

Gas Power Cycles
Week 11 Gas Power Cycles ME 300 Thermodynamics II 1 Today’s Outline • Gas turbine engines • Brayton cycle • Analysis • Example ME 300 Thermodynamics II 2 Gas Turbine Engine • Produces shaft power by GE H series power generation gas turbine. This 480-megawatt unit has a rated thermal expanding high enthalpy gas efficiency of 60% in combined cycle through a turbine configurations. • A compressor and a combustor produce the enthalpy increasing pressure and temperature, resp. • The spinning turbine rotates a shaft which drives the compressor • Remaining enthalpy can be used to drive a generator or can be expanded in a nozzle producing kinetic energy e.g. thrust ME 300 Thermodynamics II 3 Enthalpy Generation and Conversion Turbine Fuel converts enthalpy Generator to shaft power for Compressor Combustor electricity increases increases pressure Temperature Air (pv) (u) Nozzle converts enthalpy into kinetic energy ME 300 Thermodynamics II 4 Gas Turbine Engine Components http://www.stanford.edu/group/ctr/ResBriefs/temp05/schluter2.pdf www.aem.umn.edu/research/Images/pw_GasTurbine.gif ME 300 Thermodynamics II 5 Combustor Simulations ME 300 Thermodynamics II 6 Schematic • Fresh air enters compressors ~constant pressure • Air compressed to high pressure in compressor e.g. 23:1 • High pressure air is mixed with injected fuel spray in combustor raising temperatures • High enthalpy product gases expand in turbine producing shaft work to run compressor or Open cycle generator ME 300 Thermodynamics II 7 Air Standard Brayton Cycle • Model as closed cycle -
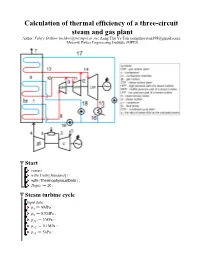
Calculation of Thermal Efficiency of a Three-Circuit Steam and Gas Plant
Calculation of thermal efficiency of a three-circuit steam and gas plant Author: Valery Ochkov ([email protected]), Aung Thu Ya Tun ([email protected]), Moscow Power Engineering Institute (MPEI) Start > > > > Steam turbine cycle Input data: > > > > > > > > > > > > > > Functions and procedures The specific enthalpy of the steam entering the turbine HPP: > (2.1) The specific enthalpy of steam at outlet of the turbine HPP: > (2.2) Specific work of HPP steam turbine: > (2.3) The temperature of the steam at the outlet of the HPP turbine: > (2.4) The specific enthalpy of steam at the point 10: > (2.5) > (2.6) The balance of the mixing streams at points 9 and 10: > (2.7) The temperature of steam at the inlet to the MPP of the turbine: > (2.8) > (2.8) > (2.9) > Specific enthalpy of water vapor at the inlet to the MPP of the turbine: > (2.10) Specific enthalpy of steam at the outlet of the turbine MPP: > (2.11) C : > (2.12) The temperature of the steam at the outlet of the MPP of the turbine: > (2.13) The specific enthalpy of steam at point 13: > (2.14) > (2.15) Balance when mixing streams at points 12 and 13: > (2.16) The temperature of steam at the inlet to the LPP of the turbine: > (2.17) > (2.18) The specific enthalpy of steam at outlet of the turbine LPP: > (2.19) Specific LPP operation of a steam turbine: > (2.8) > (2.20) The degree of dryness of the steam at the outlet of the turbine LPP: > (2.21) Condensate temperature: > (2.22) Specific enthalpy of condensate: > (2.23) The specific enthalpy of the feed water high pressure