Faculty Disclosure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abbvie Allergan Acquisition
Creating a New Diversified Biopharmaceutical Company The Combination of AbbVie and Allergan Investor Presentation June 25, 2019 NO OFFER OR SOLICITATION This presentation is not intended to and does not constitute an offer to sell or the solicitation of an offer to subscribe for or buy or an invitation to purchase or subscribe for any securities or the solicitation of any vote or approval in any jurisdiction pursuant to the acquisition or otherwise, nor shall there be any sale, issuance or transfer of securities in any jurisdiction in contravention of applicable law. In particular, this presentation is not an offer of securities for sale into the United States. No offer of securities shall be made in the United States absent registration under the U.S. Securities Act of 1933, as amended, or pursuant to an exemption from, or in a transaction not subject to, such registration requirements. Any securities issued in the acquisition are anticipated to be issued in reliance upon available exemptions from such registration requirements pursuant to Section 3(a)(10) of the U.S. Securities Act of 1933, as amended. The acquisition will be made solely by means of the Scheme Document (or, if applicable, the Takeover Offer document), which will contain the full terms and conditions of the acquisition, including details with respect to the AbbVie shareholder vote in respect of the acquisition. Any decision in respect of, or other response to, the acquisition, should be made only on the basis of the information contained in the Scheme Document. IMPORTANT ADDITIONAL INFORMATION WILL BE FILED WITH THE SEC In connection with the proposed Acquisition, Allergan will file with the Securities Exchange Commission (the “SEC”) a Proxy Statement, which will include the Scheme Document. -

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

The Covid–19 Pandemic and Haemoglobin Disorders
THE COVID–19 PANDEMIC AND HAEMOGLOBIN DISORDERS VACCINATIONS & THERAPEUTIC DRUGS An Informational Guide from the Thalassaemia International Federation (TIF) Prepared by: Dr Androulla Eleftheriou, Executive Director, TIF Last Updated: 12 May 2020 VACCINATIONS & THERAPEUTIC DRUGS Introduction It is important to note that there are currently no FDA1 or EMA2-approved or even recommended agents for the treatment of the novel coronavirus (COVID-19), for which the World Health Organization (WHO) declared as pandemic on Wednesday 11th of March 2020. Any agent being used at this time is being administered in an experimental setting under controlled conditions. Thalassaemia International Federation (TIF) has made an effort to compile a list of studies/clinical trials for treatment and vaccines, which is by no means exhaustive as this situation is extremely labile and research in this area is dramatically intensified. New information is anticipated to be added to this guide which is prepared exclusively for TIF’s global thalassemia community. The viral genome was mapped very soon as rom early January 2020 and shared globally. In February 2020, the WHO published an overview of the potential therapeutic candidates for the treatment of COVID-19. The document outlines 76 regimens that have been proposed (as of February 17, 2020) for the treatment of patients infected with the virus. Thirty-eight of these candidates are in the preclinical state with minimal information available on their proposed mechanism, uses, doses routes, or planned trials. Sixteen of the remaining regimens contain an interferon-based product. The rest include a variety of antimicrobials, corticosteroids, convalescent plasma, and biologics. The Director-General of the WHO, Mr Tedros Adhanom, stated on the 10th of April 2020, that more than 70 countries have joined WHO’s trial to accelerate research on effective treatments and 20 Institutions and companies ‘are racing to develop a vaccine’. -

Introducing the IMED Biotech Unit What Science Can Do Introduction What Science Can Do
Introducing the IMED Biotech Unit What science can do Introduction What science can do At AstraZeneca, our purpose is to push the Our IMED Biotech Unit applies its research and Our approach to R&D development capabilities and technologies to The IMED Biotech Unit plays a critical boundaries of science to deliver life-changing accelerate the progress of our pipeline. Through role in driving AstraZeneca’s success. Working together with MedImmune, medicines. We achieve this by placing science great collaboration across our three science units, our global biologics arm and Global we are confident that we can deliver the next wave Medicines Development (GMD), our at the centre of everything we do. late-stage development organisation, of innovative medicines to transform the lives of we are ensuring we deliver an innovative patients around the world. and sustainable pipeline. Pancreatic beta cells at different Eosinophil prior to Minute pieces of circulating tumour DNA stages of regeneration apoptosis (ctDNA) in the bloodstream IMED Biotech Unit MedImmune Global Medicines Development Focuses on driving scientific advances Focuses on biologics research and Focuses on late-stage development in small molecules, oligonucleotides and development in therapeutic proteins, of our innovative pipeline, transforming other emerging platforms to push the monoclonal antibodies and other next- exciting science into valued new boundaries of medical science. generation molecules to attack a range medicines and ensuring patients of diseases. around the world can access them. It’s science that compels us to push the boundaries of what is possible. We trust in the potential of ideas and pursue them, alone and with others, until we have transformed the treatment of disease. -

1:19-Cv-02226 Document #: 1 Filed: 04/01/19 Page 1 of 74 Pageid #:1
Case: 1:19-cv-02226 Document #: 1 Filed: 04/01/19 Page 1 of 74 PageID #:1 IN THE UNITED STATES DISTRICT COURT FOR THE NORTHERN DISTRICT OF ILLINOIS WELFARE PLAN OF THE INTERNATIONAL UNION OF OPERATING ENGINEERS LOCALS 137, 137A, 137B, 137C, 137R, on behalf of itself Civil Action No.______________ and all others similarly situated, CLASS ACTION Plaintiff, v. JURY TRIAL DEMANDED ABBVIE INC., ABBVIE BIOTECHNOLOGY LTD, and AMGEN INC., Defendants. CLASS ACTION COMPLAINT Case: 1:19-cv-02226 Document #: 1 Filed: 04/01/19 Page 2 of 74 PageID #:2 TABLE OF CONTENTS I. INTRODCUTION ...............................................................................................................1 II. PARTIES .............................................................................................................................3 III. JURISDICTION AND VENUE ..........................................................................................5 IV. THE REGULATORY FRAMEWORK OF BIOLOGICS ..................................................6 A. Cost Concerns Prompt Congress to Enact the Biologics Price Competition and Innovation Act ..............................................................................6 1. New biologic drug approval under the BPCIA ............................................7 2. Biosimilar drug approval under the BPCIA .................................................8 3. Biosimilar entrants’ effect on marketplace ..................................................9 B. Patent Challenges Under the BPCIA .....................................................................11 -

Effectiveness of Pfizer-Biontech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021
Morbidity and Mortality Weekly Report Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Among Hospitalized Adults Aged ≥65 Years — United States, January–March 2021 Mark W. Tenforde, MD, PhD1; Samantha M. Olson, MPH1; Wesley H. Self, MD2; H. Keipp Talbot, MD2; Christopher J. Lindsell, PhD2; Jay S. Steingrub, MD3; Nathan I. Shapiro, MD4; Adit A. Ginde, MD5; David J. Douin, MD5; Matthew E. Prekker, MD6; Samuel M. Brown, MD7; Ithan D. Peltan, MD7; Michelle N. Gong, MD8; Amira Mohamed, MD8; Akram Khan, MD9; Matthew C. Exline, MD10; D. Clark Files, MD11; Kevin W. Gibbs, MD11; William B. Stubblefield, MD2; Jonathan D. Casey, MD2; Todd W. Rice, MD2; Carlos G. Grijalva, MD2; David N. Hager, MD, PhD12; Arber Shehu, MD12; Nida Qadir, MD13; Steven Y. Chang, MD, PhD13; Jennifer G. Wilson, MD14; Manjusha Gaglani, MBBS15,16; Kempapura Murthy, MPH15; Nicole Calhoun, LMSW, MPA15; Arnold S. Monto, MD17; Emily T. Martin, PhD17; Anurag Malani, MD18; Richard K. Zimmerman, MD19; Fernanda P. Silveira, MD19; Donald B. Middleton, MD19; Yuwei Zhu, MD2; Dayna Wyatt2; Meagan Stephenson, MPH1; Adrienne Baughman2; Kelsey N. Womack, PhD2; Kimberly W. Hart2; Miwako Kobayashi, MD1; Jennifer R. Verani, MD1; Manish M. Patel, MD1; IVY Network; HAIVEN Investigators On April 28, 2021, this report was posted as an MMWR Early ≥65 years. Vaccination is a critical tool for reducing severe Release on the MMWR website (https://www.cdc.gov/mmwr). COVID-19 in groups at high risk. Adults aged ≥65 years are at increased risk for severe outcomes Randomized clinical trials of vaccines that have received an from COVID-19 and were identified as a priority group to EUA in the United States showed efficacy of 94%–95% in receive the first COVID-19 vaccines approved for use under preventing COVID-19–associated illness (4,5).§ However, an Emergency Use Authorization (EUA) in the United States hospitalization is a rare outcome among patients with (1–3). -

2019 Annual Report on Form 10-K
2 0 1 9 A n n u a l R e p o r t o n AbbVie F o r m 1 0 - K 2 0 2 0 . N Here Now o t i c e o f A n n u a l M e e t i n g & P r o x y S t a t e m e n t 2019 Annual Report on Form 10-K 2020 Notice of AbbVie 1 North Waukegan Road, North Chicago, IL 60064 U.S.A. Annual Meeting Copyright© 2020 AbbVie. All rights reserved. abbvie.com & Proxy Statement 3033_Cover.indd 1 3/18/20 6:36 PM AbbVie’s Commitment to Corporate Responsibility Stockholder Information We strive to make a remarkable impact on patients and drive sustainable growth by discovering and AbbVie Inc. Corporate Headquarters delivering a consistent stream of innovative medicines that address serious health problems. 1 North Waukegan Road North Chicago, IL 60064 IIn accordance wiitth our Priinciiplles:: 847.932.7900 abbvie.com Transforming lives Embracing diversity and inclusion Acting with integrity Serving the community Investor Relations Driving innovation Dept. ZZ05, AP34 Our Corporate Responsibility priorities are: Corporate Secretary Dept. V364, AP34 Using our expertise to Stewarding our ethical and Supporting long-term Stock Listing improve health sustainable business community strength The ticker for AbbVie’s common stock Creating real health improvement is We recognize that health is of The health of our business is is ABBV. The principal market for our mission and the premise of our fundamental importance to all people. intertwined with that of our communities. -
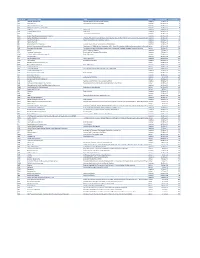
Mvx List.Pdf
MVX_CODE manufacturer_name Notes status last updated date manufacturer_id AB Abbott Laboratories includes Ross Products Division, Solvay Inactive 16-Nov-17 1 ACA Acambis, Inc acquired by sanofi in sept 2008 Inactive 28-May-10 2 AD Adams Laboratories, Inc. Inactive 16-Nov-17 3 ALP Alpha Therapeutic Corporation Inactive 16-Nov-17 4 AR Armour part of CSL Inactive 28-May-10 5 AVB Aventis Behring L.L.C. part of CSL Inactive 28-May-10 6 AVI Aviron acquired by Medimmune Inactive 28-May-10 7 BA Baxter Healthcare Corporation-inactive Inactive 28-May-10 8 BAH Baxter Healthcare Corporation includes Hyland Immuno, Immuno International AG,and North American Vaccine, Inc./acquired somInactive 16-Nov-17 9 BAY Bayer Corporation Bayer Biologicals now owned by Talecris Inactive 28-May-10 10 BP Berna Products Inactive 28-May-10 11 BPC Berna Products Corporation includes Swiss Serum and Vaccine Institute Berne Inactive 16-Nov-17 12 BTP Biotest Pharmaceuticals Corporation New owner of NABI HB as of December 2007, Does NOT replace NABI Biopharmaceuticals in this codActive 28-May-10 13 MIP Emergent BioSolutions Formerly Emergent BioDefense Operations Lansing and Michigan Biologic Products Institute Active 16-Nov-17 14 CSL bioCSL bioCSL a part of Seqirus Inactive 26-Sep-16 15 CNJ Cangene Corporation Purchased by Emergent Biosolutions Inactive 29-Apr-14 16 CMP Celltech Medeva Pharmaceuticals Part of Novartis Inactive 28-May-10 17 CEN Centeon L.L.C. Inactive 28-May-10 18 CHI Chiron Corporation Part of Novartis Inactive 28-May-10 19 CON Connaught acquired by Merieux Inactive 28-May-10 21 DVC DynPort Vaccine Company, LLC Active 28-May-10 22 EVN Evans Medical Limited Part of Novartis Inactive 28-May-10 23 GEO GeoVax Labs, Inc. -

Salix Pharmaceuticals, Inc. 2007 Annual Report and Form 10-K
2007 Annual Report and Form 10-K Advancing Treatment in GastroenterologyTM Corporate Mission Statement Salix is committed to being the leading U.S. specialty pharmaceutical Company licensing, developing and marketing innovative products to health care professionals to prevent or treat gastrointestinal disorders in patients while providing rewarding opportunities for our employees and creating exceptional value for our stockholders. To Our Stockholders Despite the December 28, 2007 approval of three generic delivery of mesalamine, or 5-ASA, beginning in the small balsalazide products by the Office of Generic Drugs, Salix bowel and continuing throughout the colon. Wilmington succeeded in making substantial advances in its business Pharmaceuticals, which licensed metoclopramide-ZYDIS to during 2007. From a product development standpoint we us, is moving forward in seeking FDA approval to market made impressive strides toward accessing both the multi- this fast-dissolving formulation. At this time, Wilmington is billion dollar irritable bowel syndrome market as well as targeting a fourth quarter 2008 approval. We believe that the hepatic encephalopathy market. We also progressed in our specialized sales force is positioned to effectively our effort to expand our presence in the inflammatory commercialize this patient-friendly formulation of this bowel disease market. On the marketing and sales side, widely-prescribed agent, if and when approved. we grew OSMOPREP® and MOVIPREP® to command a 25% We expect the product development success share of the prescription bowel cleansing market and we achieved during 2007 to be followed by commercial continued to grow XIFAXAN. On the business development success during 2008, as we anticipate receiving responses front we broadened our portfolio with the acquisitions of from the Food and Drug Administration during 2008 PEPCID OS® and metoclopramide-ZYDIS®. -

Spec Pharma M&A Transaction Multiples
SECTOR REPORT FOURTH QUARTER 2016 Disclaimer All information set forth in this report (the “Overview”) has been synthesized by Bourne Capital Partners, L.L.C. (“BP”) or was obtained from publicly available sources. BP makes no express or implied representation or warranty as to the accuracy or completeness of the information contained herein. BP expressly disclaims any and all liability that may be based on all information set forth in the Overview, errors therein, or omissions therefrom. This Overview includes certain statements, estimates and projections provided by BP with respect to anticipated future performance. Such statements, estimates and projections reflect various assumptions made by BP concerning anticipated results, which reflect significant subjective judgments made by BP and as a result, may or may not prove to be correct. There can be no assurance that such projected results are attainable or will be realized. No express or implied representations or warranties are made as to the accuracy of such statements, estimates or projections. In furnishing the Overview, BP does not undertake any obligation to provide the recipient with access to any additional information, to correct any inaccuracies that may become apparent or to update or otherwise revise this Overview. This Overview is not an offer to sell or a solicitation of an offer to purchase securities or to engage in any other transaction. BP is a North Carolina (USA) limited liability company doing business as Bourne Partners with divisions in Healthcare Merchant Banking, Alternative Assets, Management Consulting and Investment Banking. Investment Banking services are offered by Bourne Partners Securities, LLC, a registered broker dealer, Member FINRA and SIPC. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013