Salix Pharmaceuticals, Inc. 2007 Annual Report and Form 10-K
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Essex Hangar Aircrafi: Challenger (Schering Plough) Tail Number: N34CD Flight Time: 50 Minutes
This document is from the collections at the Dole Archives, University of Kansas http://dolearchives.ku.edu ... DRAFT# 1- 8/26/94 ·- Contact: Mo Taggart 703/684-7848 Jo-Anne Coe 703/845-1714 SENATOR DOLE SCHEDULE ~ AUGUST 30, 1994 -NEW YORK TUESDAY. AUGUST 30 .. 1".4 10:35 am DEPART Capitol for National Airport Ori ver: Wilbert 10:50 am ARRIVE National Airport and proceed to departing aircraft PBO: Signature 703/419·8440 10:55 am DEPART Wushington for Morristown, NJ FBO: Essex Hangar Aircrafi: Challenger (Schering Plough) Tail number: N34CD Flight time: 50 minutes Pilots: Curt Barsi John Santucci Seats: 6 Meal: None Manifest: Senator Dole Rob Lively, Schcring Plough Mike Glassner • Mark Miller Contact: Rob Lively 202/463-8809 11:45 am ARRIVE Morristown, NJ FBO: Essex Hangar 201/539-6554 11 :50 am DEPART airport for Shering Plough Headquarters Driver: Mr. Luciano's driver Drive time: 5 minutes J,ocation: l Giralda Fanns Madison, NJ 11 :55 am ARRIVE Schering Plough Headquarters 20 I /822-7440 Page 1 of 48 This document is from the collections at the Dole Archives, University of Kansas http://dolearchives.ku.edu TUESDAY, AUGUSI-.3D .1994 PAGE TWO 12:00 N- ATTEND Lunch with Bob Luciano, Chairman of Schering Plough 1:30 pm 1:30 pm DEPART Schering Plough Headquarters for airport Driver: Mr. Luciano's driver Drive time: 5 minutes 1:35 pm ARRIVE airport and proceed to departing heliocopter FBO: Essex Hangar 201/539-6554 1:40 pm DEPART Morristown, NJ for New York Helo pad: 34th Strecf Helo pad Aircraft: S-76 . -

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

2014.10.22-110Cv00528-Pfizer-Etal
IN THE UNITED STATES DISTRICT COURT FOR THE DISTRICT OF DELAWARE PFIZER INC., PHARMACIA & UPJOHN ) COMPANY, PHARMACIA & UP JOHN ) COMPANY LLC, SUGEN, INC., C.P .. ) PHARMACEUTICALS INTERNATIONAL ) C.V., PFIZER PHARMACEUTICALS LLC, ) C.A. No. 10-528-GMS and PF PRISM C.V., ) ) Plaintiffs, ) ) v. ) ) MYLAN PHARMACEUTICALS INC., ) ) Defendant. ) _______________________________ ) MEMORANDUM I. INTRODUCTION In this patent infringement action, plaintiffs Pfizer Inc., Pharmacia & Upjohn Company, Pharmacia & Upjohn Company LLC, Sugen, Inc., C.P. Pharmaceuticals International C.V., Pfizer Pharmaceuticals LLC, and PF Prism C.V. (collectively, "Pfizer") allege that pharmaceutical products proposed by defendant Mylan Pharmaceuticals Inc. ("Mylan") infringe the asserted claims of the patents-in-suit. (D.I. 1.) The court held a four-day bench trial in this matter on November 26 through November 29, 2012. (D.I. 148-151.) Presently before the court are the parties' post-trial proposed findings of fact and conclusions of law concerning the validity of the patents-in-suit, specifically whether the asserted claims are invalid as obvious under 35 U.S.C. § 103. (D.I. 152, 153.) Pursuant to Federal Rule of Civil Procedure 52( a), and after having considered the entire record in this case and the applicable law, the court concludes that: (1) all asserted claims ofthe patents-in-suit are not invalid due to obviousness; and (2) Pfizer's Rule 52(c) motion is granted, and Mylan's Rule 52( c) motion is denied. These findings of fact and conclusions oflaw are set forth in further detail below. II. FINDINGS OF FACT 1 A. The Parties 1. Plaintiff Pfizer Inc. -

FDA Listing of Authorized Generics As of July 1, 2021
FDA Listing of Authorized Generics as of July 1, 2021 Note: This list of authorized generic drugs (AGs) was created from a manual review of FDA’s database of annual reports submitted to the FDA since January 1, 1999 by sponsors of new drug applications (NDAs). Because the annual reports seldom indicate the date an AG initially entered the market, the column headed “Date Authorized Generic Entered Market” reflects the period covered by the annual report in which the AG was first reported. Subsequent marketing dates by the same firm or other firms are not included in this listing. As noted, in many cases FDA does not have information on whether the AG is still marketed and, if not still marketed, the date marketing ceased. Although attempts have been made to ensure that this list is as accurate as possible, given the volume of d ata reviewed and the possibility of database limitations or errors, users of this list are cautioned to independently verify the information on the list. We welcome comments on and suggested changes (e.g., additions and deletions) to the list, but the list may include only information that is included in an annual report. Please send suggested changes to the list, along with any supporting documentation to: [email protected] A B C D E F G H I J K L M N O P Q R S T U V X Y Z NDA Applicant Date Authorized Generic Proprietary Name Dosage Form Strength Name Entered the Market 1 ACANYA Gel 1.2% / 2.5% Bausch Health 07/2018 Americas, Inc. -

Spec Pharma M&A Transaction Multiples
SECTOR REPORT FOURTH QUARTER 2016 Disclaimer All information set forth in this report (the “Overview”) has been synthesized by Bourne Capital Partners, L.L.C. (“BP”) or was obtained from publicly available sources. BP makes no express or implied representation or warranty as to the accuracy or completeness of the information contained herein. BP expressly disclaims any and all liability that may be based on all information set forth in the Overview, errors therein, or omissions therefrom. This Overview includes certain statements, estimates and projections provided by BP with respect to anticipated future performance. Such statements, estimates and projections reflect various assumptions made by BP concerning anticipated results, which reflect significant subjective judgments made by BP and as a result, may or may not prove to be correct. There can be no assurance that such projected results are attainable or will be realized. No express or implied representations or warranties are made as to the accuracy of such statements, estimates or projections. In furnishing the Overview, BP does not undertake any obligation to provide the recipient with access to any additional information, to correct any inaccuracies that may become apparent or to update or otherwise revise this Overview. This Overview is not an offer to sell or a solicitation of an offer to purchase securities or to engage in any other transaction. BP is a North Carolina (USA) limited liability company doing business as Bourne Partners with divisions in Healthcare Merchant Banking, Alternative Assets, Management Consulting and Investment Banking. Investment Banking services are offered by Bourne Partners Securities, LLC, a registered broker dealer, Member FINRA and SIPC. -

Viatris Group Company Jurisdiction of Incorporation Legal Address Pfizer
Viatris Group Jurisdiction Legal of Company Address Incorporation Zone Industrielle, Oued Smar, Dar El Pfizer Saidal Manufacturing Algeria Beida, Algiers, Algeria Level 1, 30 The Bond, 30-34 Hickson Agila Australasia Pty Ltd Australia Road, Millers Point NSW 2000, AUSTRALIA, NSW 2000, Australia Level 1, 30 The Bond, 30-34 Hickson Alphapharm Pty. Ltd. Australia Road, Millers Point NSW 2000, AUSTRALIA, NSW 2000, Australia Level 1, 30 The Bond, 30-34 Hickson Mylan Australia Holding Pty Ltd Australia Road, Millers Point NSW 2000, AUSTRALIA, NSW 2000, Australia Level 1, 30 The Bond, 30-34 Hickson Mylan Australia Pty Limited Australia Road, Millers Point NSW 2000, AUSTRALIA, NSW 2000, Australia Level 1, 30 The Bond, 30-34 Hickson Mylan Health Pty. Ltd. Australia Road, Millers Point NSW 2000, AUSTRALIA, NSW 2000, Australia Level 17, 135-151 Clarence St, Sydney Upjohn Australia Pty Ltd. Australia NSW, 2000, Australia Huetteldorfer Strasse 299, Vienna, Arcana Arzneimittel GmbH Austria 1140, Austria MEDA Austria Holding GmbH Austria Guglgasse 15, Vienna, 1110, Austria Meda Pharma GmbH Austria Guglgasse 15, Vienna, 1110, Austria Guglgasse 15, Wien (Vienna), 1110, Mylan Österreich GmbH Austria Austria Terhulpsesteenweg 6A, Hoeilaart, Aktuapharma NV Belgium Belgium, 1560 Park Rozendal, Terhulpsesteenweg Docpharma BVBA Belgium 6A, Hoeilaart, B-1560, Belgium Terhulpsesteenweg 6A, Hoeilaart, Meda Pharma N.V. Belgium Belgium, 1560 Park Rozendal, Terhulpsesteenweg Mylan BVBA Belgium 6A, Hoeilaart, B-1560, Belgium Terhulpsesteenweg 6A, Hoeilaart, Mylan EPD BVBA Belgium Belgium, 1560 Pfizer Innovative Supply Point Hoge Wei 10, Zaventem, 1930, Belgium International BVBA Belgium Boulevard de la Plaine 17, Ixelles, Upjohn SRL Belgium 1050, Belgium 16 Par la Ville Road, Century House, Mylan Bermuda Ltd. -

Long-Term Efficacy and Safety of Etrasimod for Ulcerative Colitis
P0403 Long-term Efficacy and Safety of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study Vermeire S1, Panés J2, Chiorean M3, Peyrin-Biroulet L4, Zhang J5, Sands BE6, Lazin K5, Cabell CH5, Naik SU5, Klassen P5, Sandborn WJ7 1Department of Gastroenterology, University Hospitals Leuven, Leuven, Belgium; 2Hospital Clinic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain; 3Division of Gastroenterology, Virginia Mason Medical Center, Seattle, WA, USA; 4Department of Gastroenterology, INSERM U954, Lorraine University, Vandoeuvre-lès-Nancy, France; 5Arena Pharmaceuticals, San Diego, CA, USA; 6Icahn School of Medicine at Mount Sinai, New York, NY, USA; 7University of California San Diego, La Jolla, CA, USA Introduction Results • Etrasimod is an oral, selective, sphingosine 1-phosphate receptors 1, Patient Disposition and Characteristics • Among patients achieving clinical response, clinical remission, or endoscopic Table 2. Summary of Adverse Events During the OLE in Patients Receiving Etrasimod 2 mg in the OLE (Safety Population) 4, and 5 (S1P1, S1P4, S1P5) modulator in development for treatment of • 118 patients (84% of DB completers) entered the OLE improvements at Week 12, treatment effects were maintained at EOT in 1 immune and inflammatory-mediated diseases — 112 patients (etrasimod safety population) received etrasimod 2 mg at the majority of patients (Figure 3) Etrasimod Etrasimod • The efficacy of etrasimod as induction therapy in adult patients with any point in the OLE Figure 3. Proportion of -
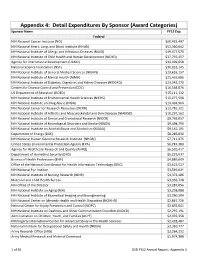
Appendix 4: Detail Expenditures by Sponsor (Award Categories) Sponsor Name FY12 Exp
Appendix 4: Detail Expenditures By Sponsor (Award Categories) Sponsor Name FY12 Exp. Federal NIH National Cancer Institute (NCI) $60,455,497 NIH National Heart, Lung, and Blood Institute (NHLBI) $52,360,642 NIH National Institute of Allergy and Infectious Diseases (NIAID) $49,477,570 NIH National Institute of Child Health and Human Development (NICHD) $37,797,457 Agency for International Development (USAID) $34,499,658 National Science Foundation (NSF) $30,033,145 NIH National Institute of General Medical Sciences (NIGMS) $29,655,157 NIH National Institute of Mental Health (NIMH) $25,435,686 NIH National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDKD) $23,042,270 Centers for Disease Control and Prevention (CDC) $16,568,876 US Department of Education (DOED) $15,111,132 NIH National Institute of Environmental Health Sciences (NIEHS) $15,077,598 NIH National Institute on Drug Abuse (NIDA) $14,494,964 NIH National Center for Research Resources (NCRR) $12,781,321 NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMSD) $10,297,162 NIH National Institute of Dental and Craniofacial Research (NIDCR) $9,769,057 NIH National Institute of Neurological Disorders and Stroke (NINDS) $9,498,790 NIH National Institute on Alcohol Abuse and Alcoholism (NIAAA) $9,161,191 Department of Energy (DOE) $8,285,830 NIH National Human Genome Research Institute (NHGRI) $7,711,975 United States Environmental Protection Agency (EPA) $6,787,380 Agency for Healthcare Research and Quality (AHRQ) $6,520,417 Department of Homeland -

Important Transaction and Tax Information Regarding the Exchange Transaction of Pharmaceutical Holdrs Trust Into Shares of Market Vectors Pharmaceutical Etf
IMPORTANT TRANSACTION AND TAX INFORMATION REGARDING THE EXCHANGE TRANSACTION OF PHARMACEUTICAL HOLDRS TRUST INTO SHARES OF MARKET VECTORS PHARMACEUTICAL ETF For electing owners (“Exchange Participants”) of Pharmaceutical HOLDRS Trust (“PPH HOLDRS”), units of PPH HOLDRS were exchanged on December 20, 2011 for shares of Market Vectors Pharmaceutical ETF. For each PPH HOLDR receipt validly tendered, Exchange Participants received 1 share of Market Vectors Pharmaceutical ETF. This exchange was effected through a series of transfers and rebalance transactions (sales transactions in certain securities underlying the PPH HOLDRS and purchase transactions in new securities for exchange into Market Vectors Pharmaceutical ETF). These transactions were authorized by the Exchange Participant and executed on their behalf. As a result of the rebalance transactions, part of this exchange may result in a taxable event and cost basis adjustments to the Exchange Participant. Provided is a breakdown of the exchange transactions and tax implications based on a ‘PPH HOLDR trading unit” (100 PPH HOLDRS receipts) 1. For each PPH HOLDR Trading Unit tendered, BNY Mellon Shareowner Services as Exchange Agent for the exchange offer, delivered the following securities per PPH HOLDR Trading Unit to BNY ConvergEx Execution Solutions LLC (“ConvergEx”), acting in its capacity transition manager in connection with the exchange offer. The total value of these securities transferred per PPH HOLDR Trading Unit, based on the December 20, 2011 market close, was $7,191.63. -

FY06 Pharmaceutical Marketing Disclosures Report
Pharmaceutical Marketing Disclosures For the period July 1, 2005 to June 30, 2006 Report of Vermont Attorney General William H. Sorrell June 26, 2007 Contact: Julie Brill Assistant Attorney General (802) 828-5479 Pharmaceutical Marketing Disclosures: Report of Vermont Attorney General William H. Sorrell on Payments to Physicians June 26, 2007 Index Page: I. Executive Summary 3 II. Description of Vermont’s Payment Disclosure Law 4 III. Amendments to Prior Pharmaceutical Marketing Disclosure Reports filed by the Vermont Attorney General's Office 5 IV. Summary of Pharmaceutical Marketing Expenditures 6 1. Total Payments of Each Pharmaceutical Manufacturer 6 2. Payments of all Manufacturers Organized by Recipient Type 7 3. Payments by Nature of Expenditure 10 4. Payments by Purpose of Expenditure 10 5. Trade Secret Declarations 11 V. Enforcement Actions 12 Appendix: Tab 1: 33 V.S.A. §2005 13 Tab 2: FY 06 Tables 17 Tab 3: FY 05 Tables as Revised 25 Tab 4: FY 04 Tables as Revised 34 Tab 5: FY 03 Tables as Revised 43 2 Pharmaceutical Marketing Disclosures: Report of Vermont Attorney General William H. Sorrell on Payments to Physicians June 26, 2007 I. Executive Summary This is the fourth report of Vermont Attorney General William H. Sorrell on Pharmaceutical Marketing Disclosures. It is based upon disclosures pertaining to payments made during the period July 1, 2005, through June 30, 2006 (FY 06) by pharmaceutical marketers, of the amount of money the companies paid during the past fiscal year on consulting and speaker fees, travel expenses, gifts, and other payments to physicians, hospitals, universities and others authorized to prescribe or dispense pharmaceutical products. -

Appendix 1: Detail Awards by Sponsor
Appendix 1: Detail Awards By Sponsor Sponsor Name FY12 Award Federal National Cancer Institute $50,418,830 National Institute of Allergy and Infectious Diseases $46,180,948 US Agency for International Development $37,208,131 National Science Foundation - Research (NSF) $32,365,368 National Institute of Child Health and Human Development (NICHD) $31,370,905 National Heart Lung and Blood Institute (NHLBI) $30,091,872 National Institute of Diabetes, Digestive and Kidney Diseases $24,989,100 National Institute of General Medicine Science $22,515,093 National Institute of Mental Health-NIH $19,346,563 National Heart Lung and Blood Institute $15,390,835 US Department of Education $15,016,549 National Institute on Drug Abuse $12,872,528 NIH National Center for Advancing Translational Sciences (NCATS) $12,546,906 National Institute of Environmental Health Sciences $12,130,116 National Institute on Alcohol Abuse and Alcoholism $11,320,987 National Institute of Arthritis Musculoskeletal Skin Disease $10,112,508 Centers for Disease Control $9,028,629 National Institute of Dental and Craniofacial Research $8,401,136 National Institute of Neurologic Disorders and Stroke $8,378,473 Bureau of Health Professions $8,085,816 US Department of Homeland Security $5,699,790 US Department of Energy $5,266,325 Centers for Disease Control and Prevention (CDC) $4,615,173 Agency for Healthcare Research and Quality $4,547,997 US Army Medical Research $4,534,927 NIH National Heart, Lung, and Blood Institute (NHLBI) $4,425,558 US Environmental Protection Agency -