Marketer1 Year of Initial Investment Investment
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Faculty Disclosure
Faculty Disclosure In accordance with the ACCME Standards for Commercial Support, course directors, planning committees, faculty and all others in control of the educational content of the CME activity must disclose all relevant financial relationships with any commercial interest that they or their spouse/partner may have had within the past 12 months. If an individual refuses to disclose relevant financial relationships, they will be disqualified from being a part of the planning and implementation of this CME activity. Owners and/or employees of a commercial interest with business lines or products relating to the content of the CME activity will not be permitted to participate in the planning or execution of any accredited activity. Nature of Relevant Financial Relationship Last Name Commercial Interest What Was Received For What Role AbbVie, Allergan/ Tobira Therapeutics Inc, Gilead Research Grant Research Balart Sciences Inc, Pfizer, Salix Pharmaceuticals AbbVie, Merck Honorarium Advisory Board Bau None N/A N/A Benz None N/A N/A AbbVie, Arbutus Biopharma, Dieterich Gilead Sciences, Inc., Bristol- Research Grant Consultant Myers Squibb, Merck Bayer HealthCare Pharmaceuticals, Gilead Sciences Honorarium Speaking, Consultant Inc. Bristol-Myers Squibb, Gilead Speaking, Advisory Sciences, Inc, Salix Honorarium Frenette Board Pharmaceuticals, Inc, Merck Intercept Pharmaceuticals Honorarium Advisor Conatus Pharmaceuticals Inc Honorarium Consulting Principle Investigator, Research Grant, Han Gilead Sciences, -

Manufacturers and Wholesalers Street
Nevada AB128 Code of Conduct Compliant Companies Manufacturers and Wholesalers Street City ST Zip 10 Edison Street LLC 13 Edison Street LLC Abbott Diabetes Care Division Abbott Diagnostic Division Abbott Electrophysiology (including Kalila Medical 2- 2016)) Abbott Laboratories 100 Abbott Park Road, Dept. EC10, Bldg. APGA-2 Abbott Park IL 60064 Abbott Medical Optics Abbott Molecular Division Abbott Nutrition Products Division Abbott Vascular Division (includes Tendyne 9-2015) AbbVie, Inc. 1 N. Waukegan Road North Chicago IL 60064 Acadia Phamaceuticals 3611 Valley Centre Drive, Suite 300 San Diego CA 92130 Accelero Health Partners, LLC Acclarent, Inc. 1525-B O'Brien Dr. Menlo Park CA 94025 Accuri Cyometers, Inc. Ace Surgical Supply, Inc. 1034 Pearl St. Brockton MA 02301 Acorda Therapeutics, Inc. 420 Sawmill River Road Ardsley NY 10532 AcriVet, Inc. Actavis W.C. Holding, Inc. Morris Corporate Center III, 400 Interpace Parkway Parsippany NJ 07054 Actavis , Inc. Actelion Pharmaceuticals US, Inc. 5000 Shoreline Court, Suite 200 S. San Francisco CA 94080 Activis 400 Interpace parkway Parsippany NJ 07054 A-Dec, Inc. 2601 Crestview Dr. Newberg OR 97132 Advanced Respiratory, Inc. Advanced Sterilization Products 33 Technology Drive Irvine CA 92618 Advanced Vision Research, Inc., dba Akorn Consumer Health Aegerion Pharmaceuticals, Inc. 101 Main Street, Suite 1850 Cambridge MA 02142 Aesculap Implant Systems, Inc. Aesculap, Inc. 3773 Corporate Parkway Center Valley PA 18034 Aesthera Corporation Afaxys, Inc. PO Box 20158 Charleston SC 29413 AGMS, Inc. Akorn (New Jersey) Inc. Page 1 of 23 Pages 2/15/2017 Nevada AB128 Code of Conduct Compliant Companies Akorn AG (formerly Excelvision AG) Akorn Animal Health, Inc. -

Q1 Pharma Sector Snapshot
SPECIALTY & GENERIC PHARMA Q1 2021 Report Market Commentary – Debt Capital Markets Debt Markets ▪ 2020 saw increased amounts of debt used in buyouts across the board, resulting in the highest debt / EBITDA Median US Buyout Multiples levels since 2014 − The increased use of debt was driven by 2H20 back- end loaded lending activity (primarily 4Q20) as 16.0x 12.7x 14.1x 12.2x 12.0x 11.6x 11.5x certainty around the U.S. election and vaccination 11.1x 10.0x 9.8x 12.0x 9.7x expectations increased 9.4x 8.6x 8.3x 8.2x 7.5x 7.8x 5.2x 6.7x 5.7x 5.6x ▪ 8.0x 5.9x As the effects of COVID now begin to diminish, debt 5.4x 4.4x 4.1x 3.7x 4.6x 4.3x 3.8x markets have seemingly recovered, signaling that 3.6x lenders have become increasingly comfortable with 4.0x 4.3x 6.9x 6.5x 6.3x 6.0x 5.9x 5.7x 5.7x 5.7x 5.7x 5.6x 5.3x 4.5x 4.4x macroeconomic and company-specific fundamentals 4.3x 0.0x 3.2x − With increased confidence, lenders are currently looking to provide strong leverage for high-quality assets, particularly ones that have proven their Debt/EBITDA Equity/EBITDA EV/EBITDA stability through the recent market downturn Source: PitchBook ▪ The spread on U.S. high-yield debt has returned to pre- Historical US High Yield Debt Effective Yield COVID levels − 4.22% current effective yield compared with a 12.0% 11.4% 11.38% effective yield on March 23, 2020 (peak of the pandemic) 9.0% ▪ We expect increased activity by lenders in 2021 due to: 6.0% 4.2% − Pent-up demand in M&A activity driven by the impact of COVID 3.0% − Limited Partner agreements and investor -

Fidelity® Select Portfolio® Pharmaceuticals Portfolio
Quarterly Holdings Report for Fidelity® Select Portfolio® Pharmaceuticals Portfolio November 30, 2020 PHR-QTLY-0121 1.810707.116 Schedule of Investments November 30, 2020 (Unaudited) Showing Percentage of Net Assets Common Stocks – 98.3% Shares Value Shares Value Biotechnology – 17.6% Bausch Health Cos., Inc. (Canada) (a) 535,300 $ 9,937,694 Biotechnology – 17.6% Bristol‑Myers Squibb Co. 1,295,680 80,850,432 Acceleron Pharma, Inc. (a) 128,800 $ 15,207,416 Catalent, Inc. (a) 75,400 7,248,956 Amgen, Inc. 137,400 30,508,296 Elanco Animal Health, Inc. (a) 172,400 5,273,716 Argenx SE (a) 17,400 4,983,924 Eli Lilly & Co. 416,961 60,730,370 Ascendis Pharma A/S sponsored ADR (a) 51,300 8,655,849 GlaxoSmithKline PLC sponsored ADR 348,300 12,813,957 Atea Pharmaceuticals, Inc. 39,800 1,326,136 Harmony Biosciences Holdings, Inc. 133,077 5,467,801 Athenex, Inc. (a) (b) 166,800 2,273,484 Horizon Therapeutics PLC (a) 316,100 22,262,923 BioNTech SE ADR (a) (b) 50,148 6,230,388 Intra‑Cellular Therapies, Inc. (a) 107,000 2,529,480 Blueprint Medicines Corp. (a) 80,300 8,678,824 Johnson & Johnson 337,850 48,880,138 ChemoCentryx, Inc. (a) 6,400 352,960 Merck & Co., Inc. 515,936 41,476,095 Galapagos Genomics NV sponsored ADR (a) 36,900 4,524,309 Nektar Therapeutics (a) (b) 368,233 6,035,339 Generation Bio Co. (b) 16,738 807,106 Novartis AG sponsored ADR 441,496 40,101,082 Generation Bio Co. -

Salix Pharmaceuticals, Inc. 2007 Annual Report and Form 10-K
2007 Annual Report and Form 10-K Advancing Treatment in GastroenterologyTM Corporate Mission Statement Salix is committed to being the leading U.S. specialty pharmaceutical Company licensing, developing and marketing innovative products to health care professionals to prevent or treat gastrointestinal disorders in patients while providing rewarding opportunities for our employees and creating exceptional value for our stockholders. To Our Stockholders Despite the December 28, 2007 approval of three generic delivery of mesalamine, or 5-ASA, beginning in the small balsalazide products by the Office of Generic Drugs, Salix bowel and continuing throughout the colon. Wilmington succeeded in making substantial advances in its business Pharmaceuticals, which licensed metoclopramide-ZYDIS to during 2007. From a product development standpoint we us, is moving forward in seeking FDA approval to market made impressive strides toward accessing both the multi- this fast-dissolving formulation. At this time, Wilmington is billion dollar irritable bowel syndrome market as well as targeting a fourth quarter 2008 approval. We believe that the hepatic encephalopathy market. We also progressed in our specialized sales force is positioned to effectively our effort to expand our presence in the inflammatory commercialize this patient-friendly formulation of this bowel disease market. On the marketing and sales side, widely-prescribed agent, if and when approved. we grew OSMOPREP® and MOVIPREP® to command a 25% We expect the product development success share of the prescription bowel cleansing market and we achieved during 2007 to be followed by commercial continued to grow XIFAXAN. On the business development success during 2008, as we anticipate receiving responses front we broadened our portfolio with the acquisitions of from the Food and Drug Administration during 2008 PEPCID OS® and metoclopramide-ZYDIS®. -

12/31/20 at 0.06% Due 01/04/21 $ 969,572 969,572 Barclays Capital, Inc
Biotechnology Fund SCHEDULE OF INVESTMENTS (Unaudited) December 31, 2020 Shares Value COMMON STOCKS† - 99.6% Biotechnology - 72.9% Amgen, Inc. 46,124 $ 10,604,830 Gilead Sciences, Inc. 137,327 8,000,671 Vertex Pharmaceuticals, Inc.* 30,030 7,097,290 Illumina, Inc.* 18,293 6,768,410 Regeneron Pharmaceuticals, Inc.* 13,496 6,520,053 Biogen, Inc.* 22,700 5,558,322 Moderna, Inc.* 49,814 5,204,069 Corteva, Inc. 125,161 4,846,234 Alexion Pharmaceuticals, Inc.* 30,030 4,691,887 Seagen, Inc.* 26,529 4,646,289 Exact Sciences Corp.* 31,204 4,134,218 Incyte Corp.* 44,634 3,882,265 Bio-Rad Laboratories, Inc. — Class A* 6,450 3,759,963 BioMarin Pharmaceutical, Inc.* 38,909 3,411,930 Alnylam Pharmaceuticals, Inc.* 25,838 3,358,165 Guardant Health, Inc.* 25,656 3,306,545 Mirati Therapeutics, Inc.* 13,253 2,910,889 Ionis Pharmaceuticals, Inc.* 48,279 2,729,695 ACADIA Pharmaceuticals, Inc.* 49,103 2,625,046 CRISPR Therapeutics AG* 16,740 2,563,061 BeiGene Ltd. ADR* 9,710 2,508,967 Acceleron Pharma, Inc.* 18,632 2,383,778 Iovance Biotherapeutics, Inc.* 50,194 2,329,002 Arrowhead Pharmaceuticals, Inc.* 30,137 2,312,412 United Therapeutics Corp.* 15,148 2,299,315 BioNTech SE ADR*,1 27,800 2,266,256 Ultragenyx Pharmaceutical, Inc.* 16,339 2,261,808 Twist Bioscience Corp.* 15,850 2,239,446 Exelixis, Inc.* 111,104 2,229,857 Novavax, Inc.*,1 19,860 2,214,589 Amicus Therapeutics, Inc.* 95,070 2,195,166 Halozyme Therapeutics, Inc.* 50,560 2,159,418 Blueprint Medicines Corp.* 18,770 2,105,056 Fate Therapeutics, Inc.* 23,040 2,095,027 Sage Therapeutics, Inc.* 23,286 2,014,472 Biohaven Pharmaceutical Holding Company Ltd.* 22,920 1,964,473 Arena Pharmaceuticals, Inc.* 25,260 1,940,726 ChemoCentryx, Inc.* 30,270 1,874,318 PTC Therapeutics, Inc.* 29,928 1,826,506 Emergent BioSolutions, Inc.* 20,360 1,824,256 Bluebird Bio, Inc.* 36,415 1,575,677 Global Blood Therapeutics, Inc.* 34,845 1,509,137 Inovio Pharmaceuticals, Inc.*,1 125,840 1,113,684 Total Biotechnology 143,863,178 Pharmaceuticals - 17.5% AbbVie, Inc. -

Spec Pharma M&A Transaction Multiples
SECTOR REPORT FOURTH QUARTER 2016 Disclaimer All information set forth in this report (the “Overview”) has been synthesized by Bourne Capital Partners, L.L.C. (“BP”) or was obtained from publicly available sources. BP makes no express or implied representation or warranty as to the accuracy or completeness of the information contained herein. BP expressly disclaims any and all liability that may be based on all information set forth in the Overview, errors therein, or omissions therefrom. This Overview includes certain statements, estimates and projections provided by BP with respect to anticipated future performance. Such statements, estimates and projections reflect various assumptions made by BP concerning anticipated results, which reflect significant subjective judgments made by BP and as a result, may or may not prove to be correct. There can be no assurance that such projected results are attainable or will be realized. No express or implied representations or warranties are made as to the accuracy of such statements, estimates or projections. In furnishing the Overview, BP does not undertake any obligation to provide the recipient with access to any additional information, to correct any inaccuracies that may become apparent or to update or otherwise revise this Overview. This Overview is not an offer to sell or a solicitation of an offer to purchase securities or to engage in any other transaction. BP is a North Carolina (USA) limited liability company doing business as Bourne Partners with divisions in Healthcare Merchant Banking, Alternative Assets, Management Consulting and Investment Banking. Investment Banking services are offered by Bourne Partners Securities, LLC, a registered broker dealer, Member FINRA and SIPC. -

Horizon Pharma Plc 2018 Irish Statutory Accounts
Horizon Pharma plc 2018 Irish Statutory Accounts (“Irish Annual Report”) CONTENTS Page DIRECTORS AND OTHER INFORMATION 2 DIRECTORS' REPORT 3 – 69 INDEPENDENT AUDITOR’S REPORT 70 – 75 CONSOLIDATED PROFIT AND LOSS ACCOUNT 76 CONSOLIDATED STATEMENT OF COMPREHENSIVE LOSS 77 CONSOLIDATED BALANCE SHEET 78 – 79 CONSOLIDATED STATEMENT OF SHAREHOLDERS’ EQUITY 80 CONSOLIDATED STATEMENT OF CASH FLOWS 81 – 82 NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS 83 – 142 PARENT COMPANY FINANCIAL STATEMENTS 143 – 153 DIRECTORS AND OTHER INFORMATION Board of Directors at December 31, 2018 Timothy P. Walbert Michael Grey William F. Daniel Jeff Himawan, Ph.D. Ronald Pauli Gino Santini James Shannon, M.D. H. Thomas Watkins Pascale Witz Secretary and Registered Office Anne-Marie Dempsey Connaught House 1st Floor 1 Burlington Road Dublin 4 D04 C5Y6 Ireland Registered Number: 507678 Auditor PricewaterhouseCoopers Chartered Accountants and Statutory Audit Firm One Spencer Dock North Wall Quay Dublin 1 D01 X9R7 Ireland Solicitor Matheson 70 Sir John Rogerson’s Quay Grand Canal Dock Dublin 2 D02 R296 Ireland 2 DIRECTORS’ REPORT The directors present their report and the audited financial statements of the Company (as defined below) for the financial year ended December 31, 2018. Basis of Presentation The Company is a public limited company formed under the laws of Ireland. The Company operates through a number of international and U.S. subsidiaries with principal business purposes to either perform research and development or manufacturing operations, serve as distributors -

Biopharma Leaders Join Trump and Thunberg in Davos
No. 3989 January 31, 2020 again a key theme at Davos, and many biopharma executives are joining the con- versation this year. These include Werner Baumann, CEO of Bayer, who spoke on a panel on 21 Janu- ary about ‘When Humankind Overrides Evolution,’ focusing on gene-editing tech- nologies for use in agriculture, food and medicine. Meanwhile Takeda CEO Chris- tophe Weber joins a discussion on shap- ing the future of health and healthcare systems on 22 January. Speaking on the gene-editing panel, Baumann said there was “less and less trust in society for the advances of tech- nology,” adding that “the only way to get beyond it is that we do a better job in terms of explaining what we are doing.” Bayer is working with CRISPR Thera- peutics on CRISPR/Cas9 gene-editing in hemophilia, ophthalmology and autoim- Biopharma Leaders Join Trump mune diseases, but also has a major stake in genetically modified seeds via its Mon- And Thunberg In Davos santo division. He called for absolute transparency ANDREW MCCONAGHIE [email protected] from corporations and governments on gene-editing technology, but also decried fter focusing on the year ahead tive to plant 1 trillion trees, an effort aimed the previous “insane dominance” that anti- in biopharma business at the J.P. at soaking up rising levels of carbon diox- GM crop campaigners have been allowed AMorgan conference in San Fran- ide in the atmosphere. to wield. cisco last week, the sector’s attention has 17-year-old environmental campaigner Others taking part include Stéphane switched to the World Economic Forum in Greta Thunberg made her own address to Bancel, Moderna Therapeutics’ chief exec- Davos, Switzerland from 21-24 January. -

Long-Term Efficacy and Safety of Etrasimod for Ulcerative Colitis
P0403 Long-term Efficacy and Safety of Etrasimod for Ulcerative Colitis: Results from the Open-label Extension of the OASIS Study Vermeire S1, Panés J2, Chiorean M3, Peyrin-Biroulet L4, Zhang J5, Sands BE6, Lazin K5, Cabell CH5, Naik SU5, Klassen P5, Sandborn WJ7 1Department of Gastroenterology, University Hospitals Leuven, Leuven, Belgium; 2Hospital Clinic de Barcelona, IDIBAPS, CIBERehd, Barcelona, Spain; 3Division of Gastroenterology, Virginia Mason Medical Center, Seattle, WA, USA; 4Department of Gastroenterology, INSERM U954, Lorraine University, Vandoeuvre-lès-Nancy, France; 5Arena Pharmaceuticals, San Diego, CA, USA; 6Icahn School of Medicine at Mount Sinai, New York, NY, USA; 7University of California San Diego, La Jolla, CA, USA Introduction Results • Etrasimod is an oral, selective, sphingosine 1-phosphate receptors 1, Patient Disposition and Characteristics • Among patients achieving clinical response, clinical remission, or endoscopic Table 2. Summary of Adverse Events During the OLE in Patients Receiving Etrasimod 2 mg in the OLE (Safety Population) 4, and 5 (S1P1, S1P4, S1P5) modulator in development for treatment of • 118 patients (84% of DB completers) entered the OLE improvements at Week 12, treatment effects were maintained at EOT in 1 immune and inflammatory-mediated diseases — 112 patients (etrasimod safety population) received etrasimod 2 mg at the majority of patients (Figure 3) Etrasimod Etrasimod • The efficacy of etrasimod as induction therapy in adult patients with any point in the OLE Figure 3. Proportion of -
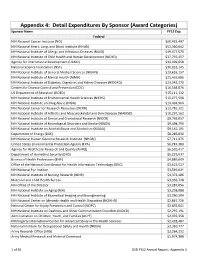
Appendix 4: Detail Expenditures by Sponsor (Award Categories) Sponsor Name FY12 Exp
Appendix 4: Detail Expenditures By Sponsor (Award Categories) Sponsor Name FY12 Exp. Federal NIH National Cancer Institute (NCI) $60,455,497 NIH National Heart, Lung, and Blood Institute (NHLBI) $52,360,642 NIH National Institute of Allergy and Infectious Diseases (NIAID) $49,477,570 NIH National Institute of Child Health and Human Development (NICHD) $37,797,457 Agency for International Development (USAID) $34,499,658 National Science Foundation (NSF) $30,033,145 NIH National Institute of General Medical Sciences (NIGMS) $29,655,157 NIH National Institute of Mental Health (NIMH) $25,435,686 NIH National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDKD) $23,042,270 Centers for Disease Control and Prevention (CDC) $16,568,876 US Department of Education (DOED) $15,111,132 NIH National Institute of Environmental Health Sciences (NIEHS) $15,077,598 NIH National Institute on Drug Abuse (NIDA) $14,494,964 NIH National Center for Research Resources (NCRR) $12,781,321 NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMSD) $10,297,162 NIH National Institute of Dental and Craniofacial Research (NIDCR) $9,769,057 NIH National Institute of Neurological Disorders and Stroke (NINDS) $9,498,790 NIH National Institute on Alcohol Abuse and Alcoholism (NIAAA) $9,161,191 Department of Energy (DOE) $8,285,830 NIH National Human Genome Research Institute (NHGRI) $7,711,975 United States Environmental Protection Agency (EPA) $6,787,380 Agency for Healthcare Research and Quality (AHRQ) $6,520,417 Department of Homeland -

Important Transaction and Tax Information Regarding the Exchange Transaction of Pharmaceutical Holdrs Trust Into Shares of Market Vectors Pharmaceutical Etf
IMPORTANT TRANSACTION AND TAX INFORMATION REGARDING THE EXCHANGE TRANSACTION OF PHARMACEUTICAL HOLDRS TRUST INTO SHARES OF MARKET VECTORS PHARMACEUTICAL ETF For electing owners (“Exchange Participants”) of Pharmaceutical HOLDRS Trust (“PPH HOLDRS”), units of PPH HOLDRS were exchanged on December 20, 2011 for shares of Market Vectors Pharmaceutical ETF. For each PPH HOLDR receipt validly tendered, Exchange Participants received 1 share of Market Vectors Pharmaceutical ETF. This exchange was effected through a series of transfers and rebalance transactions (sales transactions in certain securities underlying the PPH HOLDRS and purchase transactions in new securities for exchange into Market Vectors Pharmaceutical ETF). These transactions were authorized by the Exchange Participant and executed on their behalf. As a result of the rebalance transactions, part of this exchange may result in a taxable event and cost basis adjustments to the Exchange Participant. Provided is a breakdown of the exchange transactions and tax implications based on a ‘PPH HOLDR trading unit” (100 PPH HOLDRS receipts) 1. For each PPH HOLDR Trading Unit tendered, BNY Mellon Shareowner Services as Exchange Agent for the exchange offer, delivered the following securities per PPH HOLDR Trading Unit to BNY ConvergEx Execution Solutions LLC (“ConvergEx”), acting in its capacity transition manager in connection with the exchange offer. The total value of these securities transferred per PPH HOLDR Trading Unit, based on the December 20, 2011 market close, was $7,191.63.