Nature and Science 2013;11(12)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Project for Drainage Water Quality Control for Irrigation in Middle Nile Delta in the Arab Republic of Egypt
THE PROJECT FOR DRAINAGE WATER QUALITY CONTROL FOR IRRIGATION IN MIDDLE NILE DELTA IN THE ARAB REPUBLIC OF EGYPT Final Report March 2016 JAPAN INTERNATIONAL COOPERATION AGENCY (JICA) SANYU CONSULTANTS INC. Table of Contents Project location Map Table of Contents List of Tables List of Figures Abbreviations Chapter 1 Outline of the Project ................................................................................................... 1-1 1.1 Background: Drainage Water Reuse and Difficulties ......................................................... 1-1 1.2 Outline of Project ................................................................................................................ 1-1 1.2.1 Utilization of Proposed Master Plan ............................................................................. 1-1 1.2.2 Expected Outputs .......................................................................................................... 1-1 1.2.3 Target Area .................................................................................................................... 1-2 1.2.4 Organizations Concerned .............................................................................................. 1-2 1.2.5 Schedule and Scope of the Project ................................................................................ 1-5 1.3 Water Quality of Drainage Water in the Nile Delta Region ................................................ 1-6 1.3.1 Contamination of Drainage Water ................................................................................ -

Resettlement Policy Framework SRO Social and Resettlement Officer TOR Terms of Reference WB World Bank
RP613 Public Disclosure Authorized H olding Company for Water and Wastewater Integrated SanitationResettlement and Sewerag ePolicy Infra sFrameworktructure Pro ject (ISSIP) Public Disclosure Authorized Resettlement Po licy Framework For The Integrated Sewerage and Sanitation Infrastructure Project In Beheira, Kafr El Sheikh And Gharbeya Governorates Public Disclosure Authorized August 2007 August 2007 Public Disclosure Authorized RPF LIST OF ACRONYMS AND ABBREVIATIONS GLOSSARY LIST OF TABLES BOXES INTRODUCTION……………………………………………………………………... 1 A. Proposed Framework .................................................................................................. 2 B. Objectives of the Framework ...................................................................................... 2 C. BRIEF DESCRIPTION OF POSSIBLE REQUIRING LAND ACQUISITION AND / OR 3 RESETTLEMENT……………………………………………………………………. SECTION 1: LEGISLATIVE FRAMEWORK FOR RESETTLEMENT ....................... 4 1.1 Government of Egypt Relevant Legislation .............................................................. 4 1.2 Administrative Authority’s Decision Making Responsibilities................................. 4 1.3 Legal and Administrative Procedures for Transfer of Ownership and 6 Compensation ............................................................................................................ 1.4 Disputes...................................................................................................................... 8 1.5 Temporary Expropriation of Real Estate .................................................................. -

Detecting Methods of Ground Water Storage Changes from the Different Date Sources in Kafr
International Journal of Scientific & Engineering Research Volume 10, Issue 9, September-2019 1735 ISSN 2229-5518 Detecting Methods of Ground Water Storage Changes from the Different Date Sources in Kafr Elsheikh Governorate, Egypt. Mahmoud El-Mewafi, Fawzi Hamid Fawzi Zarzoura and Heba Basyouni Ibrahim* ABSTRACT- Gravity monitoring is used to detect the groundwater storage changes, over traditional techniques that are very costly and require strong workers, which are very difficult. The Gravity Recovery and Climate Experiment (GRACE) measures Terrestrial Water Storage (ΔTWS) for a regional area, it is the total mass of water found in the soil column (such as, surface water, soil moisture, snow, and groundwater). In this study, Grace Data was used to calculate the changes in groundwater storage (ΔGWS) in both 2005 and 2010 in Kafr El-Sheikh, Desouk and fwa by selecting four random points in each of them and calculating the terrestrial water storage from GRACE, though global land date assimilations systems (GLDAS) soil moisture was calculated and subtracted from the terrestrial Water Storage to calculate the groundwater. In this study, data on the changes in groundwater reserves were obtained by contour maps and thus the data obtained from GRACE were verified by comparing them where the maximum values and the lowest values of the differences respectively are 20.76 and -3.68 mm. Index Terms GRACE, Gravity, Traditional, GLDAS, Soil moisture, Storage —————————— —————————— 1. INTRODUCTION Ater is considered an important resource in Egypt for many From the Gravity Recovery and Climate Experiment (GRACE) water users. The next fight will be on water because of the mission {2}. -

C, Feg 19 2009
OMB No 1545-0047 dorm 990 Return of Organization Exempt From Income Tax 2007 Under section 501(c), 527, or 4947(a)(1) of the Internal Revenue Code (except black lung benefit trust or private foundation) Open to Public 3rtment of the Treasury naI Revenue Service(71 The organization may have to use a copy of this return to satisfy state reporting requirements Ins pection A For the 2007 calendar year, or tax y ear be g innin g Jul 1 , 2007 , and endin Jun 30 , 2008 B Check if applicable C Name of organization D Employer Identification Number Please use Address change IRS label Pathfinder International 53-0235320 or p not Name change or type Number and street (or P 0 box if marl is not delivered to street addr) Room/suite E Telephone number See Initial return specific 9 Galen Street 217 (617) 924-7200 Instruc- Accounting City , town or county State ZIP code + 4 F Cash Accrual Termination lions. y method IL(i Amended return Watertown MA 02472-4501 Other(specfy)0" Application pending • Section 501(c)(3) organizations and 4947(a)(1) nonexempt H and l are not applicable to section 527 organizations charitable trusts must attach a completed Schedule A H (a) Is this a group return for affiliates' q Yes No (Form 990 or 990-EZ). H (b) it 'Yes,' enter number of affiliates G Web Site:', www . p athfind.or g H (c) Are all affiliates included? q Yes q No ( if 'No,' attach a list See instructions ) J Organization type (check onl y one ) 501(c) 3 4 (insert no ) q 4947( a)(1) or 11 527 H (d) Is this a separate return tiled by an organization ruting7 K Check here If the organization is not a 509(a)(3) supporting organization and its covered by a group Yes FX] gross receipts are normally not more than $25,000 A return is not required, but if the I Grou p Exem p tion Number 0. -

Visions of a General Framework for Egypt's Cultural Policy
Visions of a General Framework for Egypt’s Cultural Policy November 2015 1 2 Table of Content Dr. Ismail Serageldin’s Introduction 5 Introduction: Support to Cultural Diversity and 7 Creativity in Egypt 1- Preliminary Overview 29 Egypt in five cultural circles 31 Our Arab Culture and the Culture of Knowledge 33 About the Egyptian Identity 37 Countering the Current Conditions 38 2- The Current Cultural State of Affairs 41 The Egyptian Cultural Society 44 Key cultural issues pertaining to the book, 46 the song, the cinema, and the theater 3- Cultural Reform in Egypt 57 Vision and Objective 57 Specific Objectives 57 About Education and Media 61 The Creative Industries 64 4- Institutions and Mechanisms 67 - Museums 68 - Libraries and the Family Libraries 69 - Ministry of Antiquities 71 - General Authority for Cultural Palaces 72 - General Egyptian Book Authority 74 - The High Council of Culture 75 - Arts Academy 75 - Visual Arts Sector 77 - Theater Section 79 - Folklor and Performance Arts Sector 80 3 - The Opera 80 - Film Industry 81 - The National Center for Traditional Crafts 84 - Scientific Societies 85 - Oral Heritage 86 - Cultural Fields and reforming their positions 86 - Dar al Kuttub and National Archives 87 - The National Translation Center 87 5- Funding 89 The Cultural Development Fund 90 Antiquities Fund 90 The Private and Public Sectors 91 Using Government Guarantee 91 6- The Digital Revolution and How to Deal with It The New Knowledge Revolution (The Seven Pillars) 93 First: Parsing, Life, and Organization 93 Second: Image and -

SYNAXARION, COPTO-ARABIC, List of Saints Used in the Coptic Church
(CE:2171b-2190a) SYNAXARION, COPTO-ARABIC, list of saints used in the Coptic church. [This entry consists of two articles, Editions of the Synaxarion and The List of Saints.] Editions of the Synaxarion This book, which has become a liturgical book, is very important for the history of the Coptic church. It appears in two forms: the recension from Lower Egypt, which is the quasi-official book of the Coptic church from Alexandria to Aswan, and the recension from Upper Egypt. Egypt has long preserved this separation into two Egypts, Upper and Lower, and this division was translated into daily life through different usages, and in particular through different religious books. This book is the result of various endeavors, of which the Synaxarion itself speaks, for it mentions different usages here or there. It poses several questions that we cannot answer with any certainty: Who compiled the Synaxarion, and who was the first to take the initiative? Who made the final revision, and where was it done? It seems evident that the intention was to compile this book for the Coptic church in imitation of the Greek list of saints, and that the author or authors drew their inspiration from that work, for several notices are obviously taken from the Synaxarion called that of Constantinople. The reader may have recourse to several editions or translations, each of which has its advantages and its disadvantages. Let us take them in chronological order. The oldest translation (German) is that of the great German Arabist F. Wüstenfeld, who produced the edition with a German translation of part of al-Maqrizi's Khitat, concerning the Coptic church, under the title Macrizi's Geschichte der Copten (Göttingen, 1845). -

Remote Area List 2018 Effective Date: 10 Jun 2018
Remote Area List 2018 Effective Date: 10 Jun 2018 A Remote Area is defined as a post code or in the absence of post code, a suburb /town name that is difficult to serve. A delivery to one of the following post codes and towns would attract a Remote Area surcharge. For Import Express shipments, a pickup from one of the same post codes and towns would attract a Remote Area surcharge. Please refer to your service guide for the surcharge amount in local currency. AFGHANISTAN 1723 - 1724 2345 3017 - 3018 3532 5184 Bagram 1727 2349 3023 3534 5186 - 5187 Bagram Ab 1731 2413 3044 3536 5191 - 5192 Bastion Airbase 1735 - 1737 2417 3050 3540 - 3541 5194 Jalalabad 1739 - 1741 2419 3060 3545 5200 - 5201 Kabul 1755 2421 3064 3550 5221 Kandahar 1757 - 1758 2426 3070 3580 5223 Kandahar Airbase 1761 2438 3080 - 3081 3586 5238 Marmal 1764 - 1765 2440 3085 3603 5244 1774 2447 3101 3606 5260 ALBANIA 1778 2451 - 2452 3103 3610 5265 Erseke 1781 - 1784 2454 3105 3624 5274 Peshkopi 1786 - 1788 2503 3116 3700 5280 Skrapar 1790 - 1793 2505 - 2506 3127 3703 5295 Tropoje 1801 2520 - 2521 3133 3705 5297 1803 2550 3138 3716 5301 ALGERIA 1806 - 1807 2553 3142 3722 5310 06200 1809 2555 3150 3740 5315 06500 1811 - 1812 2557 3153 3760 5321 06700 1814 - 1816 2559 - 2560 3158 4101 5327 09210 1837 2563 3164 4103 5330 09230 1853 - 1856 2566 3170 4107 5340 13002 1858 - 1867 2572 3174 4109 5345 15300 1901 2581 3180 4119 5350 15340 1905 2583 3187 4128 5357 19200 1907 2587 3190 4132 5360 19400 1909 2589 3196 4137 5365 21455 1911 2592 3200 4139 5372 22000 1913 2594 3206 4142 5380 -

Food Safety Inspection in Egypt Institutional, Operational, and Strategy Report
FOOD SAFETY INSPECTION IN EGYPT INSTITUTIONAL, OPERATIONAL, AND STRATEGY REPORT April 28, 2008 This publication was produced for review by the United States Agency for International Development. It was prepared by Cameron Smoak and Rachid Benjelloun in collaboration with the Inspection Working Group. FOOD SAFETY INSPECTION IN EGYPT INSTITUTIONAL, OPERATIONAL, AND STRATEGY REPORT TECHNICAL ASSISTANCE FOR POLICY REFORM II CONTRACT NUMBER: 263-C-00-05-00063-00 BEARINGPOINT, INC. USAID/EGYPT POLICY AND PRIVATE SECTOR OFFICE APRIL 28, 2008 AUTHORS: CAMERON SMOAK RACHID BENJELLOUN INSPECTION WORKING GROUP ABDEL AZIM ABDEL-RAZEK IBRAHIM ROUSHDY RAGHEB HOZAIN HASSAN SHAFIK KAMEL DARWISH AFKAR HUSSAIN DISCLAIMER: The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. CONTENTS EXECUTIVE SUMMARY...................................................................................... 1 INSTITUTIONAL FRAMEWORK ......................................................................... 3 Vision 3 Mission ................................................................................................................... 3 Objectives .............................................................................................................. 3 Legal framework..................................................................................................... 3 Functions............................................................................................................... -

Governorate Area Type Provider Name Card Specialty Address Telephone 1 Telephone 2
Governorate Area Type Provider Name Card Specialty Address Telephone 1 Telephone 2 Metlife Clinic - Cairo Medical Center 4 Abo Obaida El bakry St., Roxy, Cairo Heliopolis Metlife Clinic 02 24509800 02 22580672 Hospital Heliopolis Emergency- 39 Cleopatra St. Salah El Din Sq., Cairo Heliopolis Hospital Cleopatra Hospital Gold Outpatient- 19668 Heliopolis Inpatient ( Except Emergency- 21 El Andalus St., Behind Cairo Heliopolis Hospital International Eye Hospital Gold 19650 Outpatient-Inpatient Mereland , Roxy, Heliopolis Emergency- Cairo Heliopolis Hospital San Peter Hospital Green 3 A. Rahman El Rafie St., Hegaz St. 02 21804039 02 21804483-84 Outpatient-Inpatient Emergency- 16 El Nasr st., 4th., floor, El Nozha Cairo Heliopolis Hospital Ein El Hayat Hospital Green 02 26214024 02 26214025 Outpatient-Inpatient El Gedida Cairo Medical Center - Cairo Heart Emergency- 4 Abo Obaida El bakry St., Roxy, Cairo Heliopolis Hospital Silver 02 24509800 02 22580672 Center Outpatient-Inpatient Heliopolis Inpatient Only for 15 Khaled Ibn El Walid St. Off 02 22670702 (10 Cairo Heliopolis Hospital American Hospital Silver Gynecology and Abdel Hamid Badawy St., Lines) Obstetrics Sheraton Bldgs., Heliopolis 9 El-Safa St., Behind EL Seddik Emergency - Cairo Heliopolis Hospital Nozha International Hospital Silver Mosque, Behind Sheraton 02 22660555 02 22664248 Inpatient Only Heliopolis, Heliopolis 91 Mohamed Farid St. El Hegaz Cairo Heliopolis Hospital Al Dorrah Heart Care Hospital Orange Outpatient-Inpatient 02 22411110 Sq., Heliopolis 19 Tag El Din El Sobky st., from El 02 2275557-02 Cairo Heliopolis Hospital Egyheart Center Orange Outpatient 01200023220 Nozha st., Ard El Golf, Heliopolis 22738232 2 Samir Mokhtar st., from Nabil El 02 22681360- Cairo Heliopolis Hospital Egyheart Center Orange Outpatient 01200023220 Wakad st., Ard El Golf, Heliopolis 01225320736 Dr. -

Mapping of Schistosoma Mansoni in the Nile Delta, Egypt: Assessment
Acta Tropica 167 (2017) 9–17 Contents lists available at ScienceDirect Acta Tropica jo urnal homepage: www.elsevier.com/locate/actatropica Mapping of Schistosoma mansoni in the Nile Delta, Egypt: Assessment of the prevalence by the circulating cathodic antigen urine assay a a b c Ayat A. Haggag , Amal Rabiee , Khaled M. Abd Elaziz , Albis F. Gabrielli , d e,∗ Rehab Abdel Hay , Reda M.R. Ramzy a Ministry of Health and Population, Cairo, Egypt b Department of Community, Environmental, and Occupational Medicine, Faculty of Medicine, Ain Shams University, Egypt c Regional Advisor for Neglected Tropical Diseases, Department of Communicable Disease Prevention and Control, WHO/EMRO, Cairo, Egypt d Department of Public Health, Faculty of Medicine, Cairo University, Egypt e National Nutrition Institute, General Organisation for Teaching Hospitals and Institutes, Cairo, Egypt a r t i c l e i n f o a b s t r a c t Article history: In line with WHO recommendations on elimination of schistosomiasis, accurate identification of all areas Received 3 September 2016 of residual transmission is a key step to design and implement measures aimed at interrupting transmis- Received in revised form sion in low-endemic settings. To this purpose, we assessed the prevalence of active S. mansoni infection 18 November 2016 in five pilot governorates in the Nile Delta of Egypt by examining schoolchildren (6–15 years) using the Accepted 27 November 2016 Urine-Circulating Cathodic Antigen (Urine-CCA) cassette test; we also carried out the standard Kato- Available online 11 December 2016 Katz (KK) thick smear, the monitoring and evaluation tool employed by Egypt’s national schistosomiasis control programme. -

Copto-Arabic Literature | 1 COPTO-ARABIC LITERATURE
Copto-Arabic Literature | 1 COPTO-ARABIC LITERATURE Coptic literature per se, a subject treated elsewhere, is confined to the writings in the Coptic language during the early centuries of medieval Egyptian history when that language was the spoken language of the people as well as their only written instrument. After the ARAB CONQUEST OF EGYPT in the seventh century, the use of Coptic survived in the administrative structure of the government for some decades. Gradually, bilingual documents appeared in which Coptic and Arabic were used in parallel columns, mainly for clarification of administrative affairs to the Arab governors, who did not understand any Coptic. Then in the year A.H. 85/A.D. 705, the Muslim administration of the country decreed that Arabic be exclusively used in all administrative offices and all accounts. This revolutionary decision led ultimately to the establishment of Arabic as the accepted official language in the country—at the expense of Coptic. The state functionaries found it necessary to be proficient in the language of the conquerors in order to retain their positions in the administration as tax collectors and scribes. The Copts were very able linguists and soon mastered Arabic. In time, however, Arabic became preponderant in daily life and Coptic declined steadily, until sometime in the later Middle Ages it became defunct. As early as the tenth century, however, we begin to find works written in Arabic by noted Coptic personalities. Two major works written in classical Arabic appeared in that period. The first was a book of chronicles, Kitab al-Tawarikh, by Sa‘id ibn al-Bitriq (877-940), known as Eutychius, Melchite patriarch of Alexandria. -
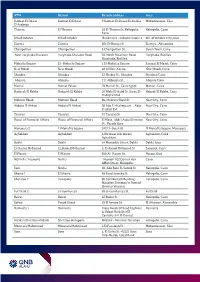
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St.