Review Article Endemic Status and Zoonotic Potential of Avian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Detecting Methods of Ground Water Storage Changes from the Different Date Sources in Kafr
International Journal of Scientific & Engineering Research Volume 10, Issue 9, September-2019 1735 ISSN 2229-5518 Detecting Methods of Ground Water Storage Changes from the Different Date Sources in Kafr Elsheikh Governorate, Egypt. Mahmoud El-Mewafi, Fawzi Hamid Fawzi Zarzoura and Heba Basyouni Ibrahim* ABSTRACT- Gravity monitoring is used to detect the groundwater storage changes, over traditional techniques that are very costly and require strong workers, which are very difficult. The Gravity Recovery and Climate Experiment (GRACE) measures Terrestrial Water Storage (ΔTWS) for a regional area, it is the total mass of water found in the soil column (such as, surface water, soil moisture, snow, and groundwater). In this study, Grace Data was used to calculate the changes in groundwater storage (ΔGWS) in both 2005 and 2010 in Kafr El-Sheikh, Desouk and fwa by selecting four random points in each of them and calculating the terrestrial water storage from GRACE, though global land date assimilations systems (GLDAS) soil moisture was calculated and subtracted from the terrestrial Water Storage to calculate the groundwater. In this study, data on the changes in groundwater reserves were obtained by contour maps and thus the data obtained from GRACE were verified by comparing them where the maximum values and the lowest values of the differences respectively are 20.76 and -3.68 mm. Index Terms GRACE, Gravity, Traditional, GLDAS, Soil moisture, Storage —————————— —————————— 1. INTRODUCTION Ater is considered an important resource in Egypt for many From the Gravity Recovery and Climate Experiment (GRACE) water users. The next fight will be on water because of the mission {2}. -

Visions of a General Framework for Egypt's Cultural Policy
Visions of a General Framework for Egypt’s Cultural Policy November 2015 1 2 Table of Content Dr. Ismail Serageldin’s Introduction 5 Introduction: Support to Cultural Diversity and 7 Creativity in Egypt 1- Preliminary Overview 29 Egypt in five cultural circles 31 Our Arab Culture and the Culture of Knowledge 33 About the Egyptian Identity 37 Countering the Current Conditions 38 2- The Current Cultural State of Affairs 41 The Egyptian Cultural Society 44 Key cultural issues pertaining to the book, 46 the song, the cinema, and the theater 3- Cultural Reform in Egypt 57 Vision and Objective 57 Specific Objectives 57 About Education and Media 61 The Creative Industries 64 4- Institutions and Mechanisms 67 - Museums 68 - Libraries and the Family Libraries 69 - Ministry of Antiquities 71 - General Authority for Cultural Palaces 72 - General Egyptian Book Authority 74 - The High Council of Culture 75 - Arts Academy 75 - Visual Arts Sector 77 - Theater Section 79 - Folklor and Performance Arts Sector 80 3 - The Opera 80 - Film Industry 81 - The National Center for Traditional Crafts 84 - Scientific Societies 85 - Oral Heritage 86 - Cultural Fields and reforming their positions 86 - Dar al Kuttub and National Archives 87 - The National Translation Center 87 5- Funding 89 The Cultural Development Fund 90 Antiquities Fund 90 The Private and Public Sectors 91 Using Government Guarantee 91 6- The Digital Revolution and How to Deal with It The New Knowledge Revolution (The Seven Pillars) 93 First: Parsing, Life, and Organization 93 Second: Image and -

Food Safety Inspection in Egypt Institutional, Operational, and Strategy Report
FOOD SAFETY INSPECTION IN EGYPT INSTITUTIONAL, OPERATIONAL, AND STRATEGY REPORT April 28, 2008 This publication was produced for review by the United States Agency for International Development. It was prepared by Cameron Smoak and Rachid Benjelloun in collaboration with the Inspection Working Group. FOOD SAFETY INSPECTION IN EGYPT INSTITUTIONAL, OPERATIONAL, AND STRATEGY REPORT TECHNICAL ASSISTANCE FOR POLICY REFORM II CONTRACT NUMBER: 263-C-00-05-00063-00 BEARINGPOINT, INC. USAID/EGYPT POLICY AND PRIVATE SECTOR OFFICE APRIL 28, 2008 AUTHORS: CAMERON SMOAK RACHID BENJELLOUN INSPECTION WORKING GROUP ABDEL AZIM ABDEL-RAZEK IBRAHIM ROUSHDY RAGHEB HOZAIN HASSAN SHAFIK KAMEL DARWISH AFKAR HUSSAIN DISCLAIMER: The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. CONTENTS EXECUTIVE SUMMARY...................................................................................... 1 INSTITUTIONAL FRAMEWORK ......................................................................... 3 Vision 3 Mission ................................................................................................................... 3 Objectives .............................................................................................................. 3 Legal framework..................................................................................................... 3 Functions............................................................................................................... -

Governorate Area Type Provider Name Card Specialty Address Telephone 1 Telephone 2
Governorate Area Type Provider Name Card Specialty Address Telephone 1 Telephone 2 Metlife Clinic - Cairo Medical Center 4 Abo Obaida El bakry St., Roxy, Cairo Heliopolis Metlife Clinic 02 24509800 02 22580672 Hospital Heliopolis Emergency- 39 Cleopatra St. Salah El Din Sq., Cairo Heliopolis Hospital Cleopatra Hospital Gold Outpatient- 19668 Heliopolis Inpatient ( Except Emergency- 21 El Andalus St., Behind Cairo Heliopolis Hospital International Eye Hospital Gold 19650 Outpatient-Inpatient Mereland , Roxy, Heliopolis Emergency- Cairo Heliopolis Hospital San Peter Hospital Green 3 A. Rahman El Rafie St., Hegaz St. 02 21804039 02 21804483-84 Outpatient-Inpatient Emergency- 16 El Nasr st., 4th., floor, El Nozha Cairo Heliopolis Hospital Ein El Hayat Hospital Green 02 26214024 02 26214025 Outpatient-Inpatient El Gedida Cairo Medical Center - Cairo Heart Emergency- 4 Abo Obaida El bakry St., Roxy, Cairo Heliopolis Hospital Silver 02 24509800 02 22580672 Center Outpatient-Inpatient Heliopolis Inpatient Only for 15 Khaled Ibn El Walid St. Off 02 22670702 (10 Cairo Heliopolis Hospital American Hospital Silver Gynecology and Abdel Hamid Badawy St., Lines) Obstetrics Sheraton Bldgs., Heliopolis 9 El-Safa St., Behind EL Seddik Emergency - Cairo Heliopolis Hospital Nozha International Hospital Silver Mosque, Behind Sheraton 02 22660555 02 22664248 Inpatient Only Heliopolis, Heliopolis 91 Mohamed Farid St. El Hegaz Cairo Heliopolis Hospital Al Dorrah Heart Care Hospital Orange Outpatient-Inpatient 02 22411110 Sq., Heliopolis 19 Tag El Din El Sobky st., from El 02 2275557-02 Cairo Heliopolis Hospital Egyheart Center Orange Outpatient 01200023220 Nozha st., Ard El Golf, Heliopolis 22738232 2 Samir Mokhtar st., from Nabil El 02 22681360- Cairo Heliopolis Hospital Egyheart Center Orange Outpatient 01200023220 Wakad st., Ard El Golf, Heliopolis 01225320736 Dr. -

Mapping of Schistosoma Mansoni in the Nile Delta, Egypt: Assessment
Acta Tropica 167 (2017) 9–17 Contents lists available at ScienceDirect Acta Tropica jo urnal homepage: www.elsevier.com/locate/actatropica Mapping of Schistosoma mansoni in the Nile Delta, Egypt: Assessment of the prevalence by the circulating cathodic antigen urine assay a a b c Ayat A. Haggag , Amal Rabiee , Khaled M. Abd Elaziz , Albis F. Gabrielli , d e,∗ Rehab Abdel Hay , Reda M.R. Ramzy a Ministry of Health and Population, Cairo, Egypt b Department of Community, Environmental, and Occupational Medicine, Faculty of Medicine, Ain Shams University, Egypt c Regional Advisor for Neglected Tropical Diseases, Department of Communicable Disease Prevention and Control, WHO/EMRO, Cairo, Egypt d Department of Public Health, Faculty of Medicine, Cairo University, Egypt e National Nutrition Institute, General Organisation for Teaching Hospitals and Institutes, Cairo, Egypt a r t i c l e i n f o a b s t r a c t Article history: In line with WHO recommendations on elimination of schistosomiasis, accurate identification of all areas Received 3 September 2016 of residual transmission is a key step to design and implement measures aimed at interrupting transmis- Received in revised form sion in low-endemic settings. To this purpose, we assessed the prevalence of active S. mansoni infection 18 November 2016 in five pilot governorates in the Nile Delta of Egypt by examining schoolchildren (6–15 years) using the Accepted 27 November 2016 Urine-Circulating Cathodic Antigen (Urine-CCA) cassette test; we also carried out the standard Kato- Available online 11 December 2016 Katz (KK) thick smear, the monitoring and evaluation tool employed by Egypt’s national schistosomiasis control programme. -
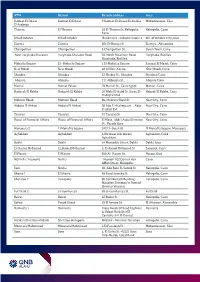
ATM Branch Branch Address Area Gameat El Dowal El
ATM Branch Branch address Area Gameat El Dowal Gameat El Dowal 9 Gameat El-Dewal El-Arabia Mohandessein, Giza El Arabeya Thawra El-Thawra 18 El-Thawra St. Heliopolis, Heliopolis, Cairo Cairo 6th of October 6th of October Banks area - industrial zone 4 6th of October City, Giza Zizenia Zizenia 601 El-Horaya St Zizenya , Alexandria Champollion Champollion 5 Champollion St., Down Town, Cairo New Hurghada Sheraton Hurghada Sheraton Road 36 North Mountain Road, Hurghada, Red Sea Hurghada, Red Sea Mahatta Square El - Mahatta Square 1 El-Mahatta Square Sarayat El Maadi, Cairo New Maadi New Maadi 48 Al Nasr Avenu New Maadi, Cairo Shoubra Shoubra 53 Shobra St., Shoubra Shoubra, Cairo Abassia Abassia 111 Abbassia St., Abassia Cairo Manial Manial Palace 78 Manial St., Cairo Egypt Manial , Cairo Hadayek El Kobba Hadayek El Kobba 16 Waly El-Aahd St, Saray El- Hdayek El Kobba, Cairo Hadayek Mall Makram Ebeid Makram Ebeid 86, Makram Ebeid St Nasr City, Cairo Abbass El Akkad Abbass El Akkad 20 Abo El Ataheya str. , Abas Nasr City, Cairo El akad Ext Tayaran Tayaran 32 Tayaran St. Nasr City, Cairo House of Financial Affairs House of Financial Affairs El Masa, Abdel Azziz Shenawy Nasr City, Cairo St., Parade Area Mansoura 2 El Mohafza Square 242 El- Guish St. El Mohafza Square, Mansoura Aghakhan Aghakhan 12th tower nile towers Aghakhan, Cairo Aghakhan Dokki Dokki 64 Mossadak Street, Dokki Dokki, Giza El- Kamel Mohamed El_Kamel Mohamed 2, El-Kamel Mohamed St. Zamalek, Cairo El Haram El Haram 360 Al- Haram St. Haram, Giza NOZHA ( Triumph) Nozha Triumph.102 Osman Ebn Cairo Affan Street, Heliopolis Safir Nozha 60, Abo Bakr El-Seddik St. -

Decree-Law of the President of the Arab Republic of Egypt No
The Official Gazette Issue no. 51 (bis.) on December 21st 2014 Page (2) Decree-Law of the President of the Arab Republic of Egypt no. 202/2014 Concerning Electoral Districting for the Elections of the House of Representatives The President of the Republic Having perused: the Constitution; Decree-Law no. 45/2014 on the regulation of the Exercise of Political Rights; Decree-Law no. 46/2014 on the House of Representatives; Upon consulting the High Elections Committee; Upon the approval of the Council of Ministers; and On the basis of the opinion of the Council of State; Decided on the following Law: Article 1 The provisions of the attached law shall apply with regard to the first elections for the House of Representatives that is held after its entry into force and any related by-elections. Any provision contradicting the provisions of the attached law is hereby abolished. Article 2 The Arab Republic of Egypt shall be divided into 237 electoral districts (constituencies) under the Individual-Seat system, and 4 constituencies under the lists system. Article 3 The scope and units of each constituency and the number of seats allocated thereto, and to each Governorate, shall be pursuant to the attached tables, in a manner which observes the fair representation of the population and Governorates and the equitable representation of voters. Article 4 This decree-law and the tables attached thereto shall be published in the Official Gazette and shall enter into force as of the day following its date of publication. Issued at the Presidency of the Republic on the 29th of Safar, 1436 A.H. -

Ahmed H. Zewail
Ahmed H. Zewail - Commemoration Professor Ahmed Hassan Zewail, Linus Pauli Professor at the California Institute of Technology (Caltech), passed away on August 2, 2016 at his home near Pasadena, California, after losing a battle with cancer. Ahmed Zewail was awarded the 1999 Nobel Prize in Chemistry as the sole winner for his pioneering work on ultrafast chemistry. He became the first science Nobel Prize winner from the Arabic-speaking world. As a native Egyptian, he was honored with the Order of the Nile, the highest honor the State of Egypt can bestow, and received a military funeral in his native country. In his research, he employed ultrashort laser pulses to develop stroboscopic pump-probe techniques on a femtosecond time scale. In this way, he made it possible to observe the extremely fast dynamics of molecular matter in slow motion and to study elementary processes in complex systems, including bio-molecules. With a shutter time a million times faster than ordinary cameras, it becomes possible to capture the motion of atoms and to resolve transient states during chemical reactions. The observation of such phenomena and the development of appropriate models has led to a deeper understanding of the motion and forces in the microcosm of atoms and molecules, with applications ranging from quantum chemistry to molecular biology. His impressively elegant experiments revealed the important role of coherence much beyond what had long been assumed. With the creation of the new field femtochemistry, Ahmed Zewail has left a towering scientific legacy that will inspire many future discoveries. More recently, Professor Zewail and his team pioneered the powerful technique of 4D microscopy that uses diffraction of ultrafast pulses of electrons to track reactions in space and time on the atomic scale. -

1St Report on the Climatology of Nile Delta, Egypt
First Report Climatology of Nile Delta, Egypt Prepared by Dr. Ashraf Zaki, Egyptian Meteorological Authority (EMA) Dr Atef Swelam, IWLMP, ICARDA January 2017 1st Report on the Climatology of Nile Delta, Egypt The Climatology of Nile Delta, Egypt 1. Introduction Weather is the condition of the atmosphere over a brief period of time. For example, we speak of today’s weather or the weather this week. Climate represents the composite of day-to-day weather over a longer period of time. Climate is usually defined by what is expected or “normal”, which climatologists traditionally interpret as the 30-year average. By itself, “normal” can be misleading unless we also understand the concept of variability. A climatologist attempts to discover and explain the impacts of climate so that society can plan its activities, design its buildings and infrastructure, and anticipate the effects of adverse conditions. Although climate is not weather, it is defined by the same terms, such as temperature, precipitation, wind, and solar radiation. The misconception that weather is usually normal becomes a serious problem when you consider that weather, in one form or another, is the source of water for irrigation, drinking, power supply, industry, wildlife habitat, and other uses. To ensure that our water supply, livelihoods, and lives are secure, it is essential that planners anticipate variation in weather, and that they recognize that drought and flood are both inevitable parts of the normal range of weather. Over Egypt and North Africa, the decrease in annual precipitation that is predicted in the 21st century will exacerbate these effects, particularly in semiarid and arid regions that rely on irrigation for crop growth. -

Rousettus Aegyptiacus (Pteropodidae) in the Palaearctic: List of Records and Revision of the Distribution Range*
Vespertilio 15: 3–36, 2011 ISSN 1213-6123 Rousettus aegyptiacus (Pteropodidae) in the Palaearctic: list of records and revision of the distribution range* Petr BENDA1,2, Mounir ABI-SAID3, Tomáš Bartonička4, Raşit BILGIN5, Kaveh FAIZOLAHI6, Radek K. Lučan2, Haris NICOLAOU7, Antonín REITER8, Wael M. SHOHDI9, Marcel UHRIN10 & Ivan Horáček2 1 Department of Zoology, National Museum (Natural History), Václavské nám. 68, CZ–115 79 Praha 1, Czech Republic; [email protected] 2 Department of Zoology, Faculty of Science, Charles University, Viničná 7, CZ–128 44 Praha 2, Czech Republic 3 Department of Biology, American University of Beirut, Riadh El Solh 1107, 2020 Beirut, Lebanon 4 Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ–611 37 Brno, Czech Republic 5 Institute of Environmental Sciences, Bogazici University, Bebek, TR–34342 Istanbul, Turkey 6 Mohitban Society, 2010 Mitra Alley, Shariati Street, Tehran, 1963816163, Iran 7 Forestry Department, Ministry of Agriculture, Natural Resources and Environment, Louki Akrita 24, Nicosia, Cyprus 8 South Moravian Museum in Znojmo, Přemyslovců 8, CZ–669 45 Znojmo, Czech Republic 9 Nature Conservation Egypt & Department of Zoology, Faculty of Science, Al-Azhar University, Cairo, Egypt 10 Institute of Biology and Ecology, P. J. Šafárik University, Moyzesova 11, SK–040 01 Košice, Slovakia Abstract. The populations of Rousettus aegyptiacus inhabiting the Palaearctic part of the species range represent the only offshoot of the family Pteropodidae beyond tropics. In this contribution, we revised distribution status of the species in different parts of its Palaearctic range, re-examined the literature data and supplemented them with an extensive set of original records obtained in the field during the last two decades. -

National Maternal Mortality Study: Egypt 2000
2 THE NATIONAL MATERNAL MORTALITY STUDY: EGYPT 2000 REPORT OF FINDINGS AND CONCLUSIONS JUNE 2001 3 © June 2001 Directorate of Maternal and Child Health Care Ministry of Health and Population, Egypt 4 The National Maternal Mortality Study: Egypt 2000 was conducted by the Directorate of Maternal and Child Health Care, Ministry of Health and Population, under the Healthy Mother/Healthy Child Project in collaboration with the United States Agency for International Development and its technical assistance contractor, John Snow, Inc. This publication was made possible through support provided by the Office of Health and Population, United States Agency for International Development/Egypt, under the terms of Contract No. 263-C-00-98-00041- 00 with John Snow, Inc. (JSI). The opinions expressed herein are those of the authors and do not necessarily reflect the views of the United States Agency for International Development or John Snow, Inc. Foreword The National Maternal Mortality Study: Egypt, 2000 is the second national study of maternal mortality to take place in Egypt. I requested that this study be repeated to measure the degree of reduction in maternal mortality since the first study (1992/93), which was conducted eight years ago. Both studies were conducted by the Ministry of Health and Population, Directorate of Maternal and Child Health Care with help from the Central Agency for Public Mobilization and Statistics (CAPMAS). Support to fund this study was provided by the Ministry of Health and Population and the United States Agency for International Development (USAID), with technical support provided by USAID contractor John Snow, Inc. It is with great pleasure that I congratulate all of my MOHP colleagues on their accomplishments and contributions to lowering the national maternal mortality ratio by over 50% (from 174 to 84 per 100,000 live births) in the past eight years. -

Proposal Evaluation
CONNECTIVITY REQUIREMENTS CRA MODERNIZATION PROJECT Commercial Registry December 14, 2009 This publication was produced for review by the United States Agency for International Development. It was prepared by Pedro Valdes. CONNECTIVITY REQUIREMENTS CRA MODERNIZATION PROJECT Commercial Registry TECHNICAL ASSISTANCE FOR POLICY REFORM II CONTRACT NUMBER: 263-C-00-05-00063-00 DELOITTE CONSULTING LLP USAID/EGYPT POLICY AND PRIVATE SECTOR OFFICE DECEMBER 14, 2009 AUTHOR: PEDRO VALDES DISCLAIMER: The author’s views expressed in this publication do not necessarily reflect the views of the United States Agency for International Development or the United States Government. CONTENTS 1.0 INTRODUCTION .......................................................................................... 1 1.1 Assumptions ................................................................................................................. 1 1.2 Bandwidth Estimation ................................................................................................... 1 1.3 Estimation Approach .................................................................................................... 2 2.0 BANDWIDTH PER OFFICES ...................................................................... 3 2.1 Bandwidth Requirements by Priority List ...................................................................... 3 2.2 Summary Implementation Priorities and Backup Lines Requirements and BW ........................................................................................................................