Occupational Exposure to Organophosphorus Pesticides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Downloads/DL Praevention/Fachwissen/Gefahrstoffe/TOXIKOLOGI SCHE BEWERTUNGEN/Bewertungen/Toxbew072-L.Pdf
Distribution Agreement In presenting this thesis or dissertation as a partial fulfillment of the requirements for an advanced degree from Emory University, I hereby grant to Emory University and its agents the non-exclusive license to archive, make accessible, and display my thesis or dissertation in whole or in part in all forms of media, now or hereafter known, including display on the world wide web. I understand that I may select some access restrictions as part of the online submission of this thesis or dissertation. I retain all ownership rights to the copyright of the thesis or dissertation. I also retain the right to use in future works (such as articles or books) all or part of this thesis or dissertation. Signature: _____________________________ ______________ Jedidiah Samuel Snyder Date Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Master of Public Health Global Environmental Health _________________________________________ P. Barry Ryan, Ph.D. Committee Chair _________________________________________ Eugene Demchuk, Ph.D. Committee Member _________________________________________ Paige Tolbert, Ph.D. Committee Member Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. Snyder Bachelor of Science in Engineering, B.S.E. The University of Iowa 2010 Thesis Committee Chair: P. Barry Ryan, Ph.D. An abstract of A thesis submitted to the Faculty of the Rollins School of Public Health of Emory University in partial fulfillment of the requirements for the degree of Master of Public Health in Global Environmental Health 2015 Abstract Statistical analysis of concentration-time extrapolation factors for acute inhalation exposures to hazardous substances By Jedidiah S. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

Metabolism of Methiocarb and Carbaryl by Rat and Human Livers and Plasma, and Effect on Their PXR, CAR and Pparα Activities
The Journal of Toxicological Sciences (J. Toxicol. Sci.) 677 Vol.41, No.5, 677-691, 2016 Original Article Metabolism of methiocarb and carbaryl by rat and human livers and plasma, and effect on their PXR, CAR and PPARα activities Chieri Fujino1, Yuki Tamura2, Satoko Tange1, Hiroyuki Nakajima3,4, Seigo Sanoh1, Yoko Watanabe2, Naoto Uramaru2, Hiroyuki Kojima5, Kouichi Yoshinari3,4, Shigeru Ohta1 and Shigeyuki Kitamura2 1Graduate School of Biomedical and Health Sciences, Hiroshima University, Kasumi 1-2-3, Minami-ku, Hiroshima 734-8553, Japan 2Nihon Pharmaceutical University, Komuro 10281, Ina-machi, Kitaadachi-gun, Saitama 362-0806, Japan 3Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aramaki-Aoba, Aoba-ku, Sendai 980-8578, Japan 4School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526, Japan 5Hokkaido Institute of Public Health, Kita-19, Nishi-12, Kita-ku, Sapporo 060-0819, Japan (Received April 14, 2016; Accepted August 2, 2016) ABSTRACT — The oxidative, reductive, and hydrolytic metabolism of methiocarb and the hydrolyt- ic metabolism of carbaryl by liver microsomes and plasma of rats or humans were examined. The effects of the metabolism of methiocarb and carbaryl on their nuclear receptor activities were also examined. When methiocarb was incubated with rat liver microsomes in the presence of NADPH, methiocarb sul- foxide, and a novel metabolite, methiocarb sulfone were detected. Methiocarb sulfoxide was oxidized to the sulfone by liver microsomes and reduced back to methiocarb by liver cytosol. Thus, the intercon- version between methiocarb and the sulfoxide was found to be a new metabolic pathway for methiocarb by liver microsomes. -

Acutely Toxic Chemical List
EPA: Acutely Toxic Chemicals List United States Environmental Protection Agency (EPA) Acutely Toxic Chemical Name EPA Waste Code CAS # Acetaldehyde, chloro- P023 107-20-0 Acetamide, N-(aminothioxomethyl)- P002 591-08-2 Acetamide, 2-fluoro- P057 640-19-7 Acetic acid, fluoro-, sodium salt P058 62-74-8 1-Acetyl-2-thiourea P002 591-08-2 Acrolein P003 107-02-8 Aldicarb P070 116-06-3 Aldicarb sulfone. P203 1646-88-4 Aldrin P004 309-00-2 Allyl alcohol P005 107-18-6 Aluminum phosphide (R,T) P006 20859-73-8 5-(Aminomethyl)-3-isoxazolol P007 2763-96-4 4-Aminopyridine P008 504-24-5 Ammonium picrate (R) P009 131-74-8 Ammonium vanadate P119 7803-55-6 Argentate(1-), bis(cyano-C)-, potassium P099 506-61-6 Arsenic acid H3AsO4 P010 7778-39-4 Arsenic oxide As2 O3 P012 1327-53-3 Arsenic oxide As2O5 P011 1303-28-2 Arsenic pentoxide P011 1303-28-2 Arsenic trioxide P012 1327-53-3 Arsine, diethyl- P038 692-42-2 Arsonous dichloride, phenyl- P036 696-28-6 Aziridine P054 151-56-4 Aziridine, 2-methyl- P067 75-55-8 Barium cyanide P013 542-62-1 Benzenamine, 4-chloro- P024 106-47-8 Benzenamine, 4-nitro- P077 100-01-6 Benzene, (chloromethyl)- P028 100-44-7 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P042 51-43-4 Benzeneethanamine, alpha, alpha-dimethyl- P046 122-09-8 Updated September 2021 T:\Documentation\EHS-Updates\Acute Waste Codes.docx CONTINUED: Liquid Nitrogen Handling and Use United States Environmental Protection Agency (EPA) Acutely Toxic Chemical Name EPA Waste Code CAS # Benzenethiol P014 108-98-5 7-Benzofuranol, 2,3-dihydro-2,2-dimethyl-, methylcarbamate. -

Residues of Carbosulfan and Its Carbofuran Metabolites and 3-Hydroxy-Carbofuran in Oranges1, 2
230 RESIDUES OF CARBOSULFAN AND ITS CARBOFURAN METABOLITES AND 3-HYDROXY-CARBOFURAN IN ORANGES1, 2 MARCOS JOSÉ TREVISAN3, GILBERTO CASADEI DE BAPTISTA4, LUIZ ROBERTO PIMENTEL TREVIZAN4, GERALDO PAPA5 ABSTRACT – The objectives of this study were to evaluate the residues of the insecticide carbosulfan and its carbofuran metabolites and 3- hydroxy-carbofuran in orange compartments (whole fruit, bagasse and juice) and comparison between the residual levels found in fruits with the maximum residue level and the safety interval established by the Brazilian legislation. Two field experiments were carried out, both with the following treatments: a-check; b-one application of 10 g of carbosulfan . 100 L-1 of water; c-one application with twice the rate applied in treatment b; d-four applications with the same rate applied in treatment b. Samples were taken at (-1), zero, 1, 3, 7, 14, 21 and 28 days after the last or unique application. The quantitative determinations were done by gas chromatography technique, using a nitrogen-phosphorus detector. The carbosulfan metabolism to its carbofuran metabolite was rapid (3 days), being both analytes concentrated in the bagasse (peel + flavedo + albedo). However, the metabolism of carbofuran to 3-hydroxy-carbofuran was of low intensity or this metabolite was quickly dissipated. Carbosulfan residues and its metabolites did not penetrate into the fruit, thus not contaminating the juice. The use of the pesticide was adequate, with respect to fruit consumption, in relation to the Brazilian legislation. Index -

Versatile New Class of Polymer for Natural and Synthetic Ruber Gloves Coating
9thInternational Rubber Glove Conference & Exhibition 2018 VERSATILE NEW CLASS OF POLYMER FOR NATURAL AND SYNTHETIC RUBER GLOVES COATING Dr. Shashidhar V. Govindaraju, Mr. Chetan Kulkarni Apcotex Industries Limited India And Mr. Khoo Siong Hui, Le Inoova Sdn Bhd, Malaysia ABSTRACT Powder coating and chlorination are the widely used processes for achieving good donning properties although polymer coating is also in practice with limited reach. With many restrictions being imposed on powder coated gloves, chlorination process is expected to assume bigger role although it poses many challenges like plant corrosion and health hazard. Taking the environmental restrictions in to consideration the future seems to be with only polymer coating (Polyacrylic or Polyurethane), which is very compatible with both glove and environment. This study introduces a new class of polymer, namely specialty carboxylated Styrene- Butadiene latex, which is widely used in various coating applications, for its long-lasting donning performance. It outperforms Acrylic emulsion for its higher binding strength and elasticity that makes it more suitable for coating both Natural rubber gloves and Synthetic rubber gloves. While matching Polyurethane coating properties it possesses higher versatility in formulation tweaking for vast application conditions, yet providing better cost effectiveness. Relevant research data is presented for the excellent donning performance by the new class of polymer for both Natural rubber gloves (NR) and Nitrile rubber gloves (NBR) gloves. BACKGROUND It is known that rubber gloves are extensively used in various fields which include housekeeping, medical, food and the like for seeking protection against infection, contagious disease, chemicals, solvents, chemotherapy drugs, cuts, bruises and static electricity. -

Pesticide Residues : Maximum Residue Limits
THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives ICS 67.040 ISBN UNOFFICAL TRANSLATION THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives 50 Phaholyothin Road, Ladyao, Chatuchak, Bangkok 10900 Telephone (662) 561 2277 Fascimile: (662) 561 3357 www.acfs.go.th Published in the Royal Gazette, Announcement and General Publication Volume 131, Special Section 32ง (Ngo), Dated 13 February B.E. 2557 (2014) (2) Technical Committee on the Elaboration of the Thai Agricultural Standard on Maximum Residue Limits for Pesticide 1. Mrs. Manthana Milne Chairperson Department of Agriculture 2. Mrs. Thanida Harintharanon Member Department of Livestock Development 3. Mrs. Kanokporn Atisook Member Department of Medical Sciences, Ministry of Public Health 4. Mrs. Chuensuke Methakulawat Member Office of the Consumer Protection Board, The Prime Minister’s Office 5. Ms. Warunee Sensupa Member Food and Drug Administration, Ministry of Public Health 6. Mr. Thammanoon Kaewkhongkha Member Office of Agricultural Regulation, Department of Agriculture 7. Mr. Pisan Pongsapitch Member National Bureau of Agricultural Commodity and Food Standards 8. Ms. Wipa Thangnipon Member Office of Agricultural Production Science Research and Development, Department of Agriculture 9. Ms. Pojjanee Paniangvait Member Board of Trade of Thailand 10. Mr. Charoen Kaowsuksai Member Food Processing Industry Club, Federation of Thai Industries 11. Ms. Natchaya Chumsawat Member Thai Agro Business Association 12. Mr. Sinchai Swasdichai Member Thai Crop Protection Association 13. Mrs. Nuansri Tayaputch Member Expert on Method of Analysis 14. -
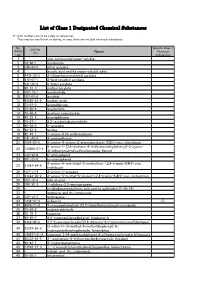
List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde -

Fluoroacetate Toxicity Hanover, New Hampshire 03755
Gordon W. Gribble' Dartmouth College Fluoroacetate Toxicity Hanover, New Hampshire 03755 Under the stress of World War I1 chemists in England, Table 1. Toxicities of MFA and Other Toxic Compoundso Germany and their allied countries sought to develop chemicals (independently, of course!) which would inca- pacitate, maim, or kill the enemy. These remarkably suc- cessful researches led to the synthesis and large-scale pro- MFA ... 6 duction of several types of warfare agents: nerve gases, FCHzCOINa 0.2 . mustard gas 0.7 . vesicant agents, tear gases, harassing compounds, and, parathion 10 . perhaps the most frightening of all, water poisons. strychnine 5 0.5 For the latter kind of chemical agent it can be easily NaCN ... 10 envisaged that a secret agent could poison the water sup- DFP (nerve agent) . 4 .nlv . of a large" enemv". ~onulace. with hut a small amount of OTaken from several sources. a toxic chemical. The requirements for a water poison are The dose required to kill 50% of the animals by subcutaneous strineent: it should he colorless. odorless. soluble. stable. injection. and rhighly toxic, preferably with a delayed action to pre: Table 2. Selected Physical Properties of MFAa vent early detection. It therefore must have come as quite a surprise to chemists in England, Germany, and Poland Property Value when they discovered independently during the early stag- hoilina ~oint 104' (760 mm) es of the war that a simple derivative of acetic acid fulfills meltingpoint -32' all of the above criteria for an ideal water poison! water solubility 15% This compound is methyl fluoroacetate (MFA) and it, odor faintlv fruitv at 10 DDm along with fluoroacetic acid (FA) and 2-fluoroethanol, represents one of the most toxic classes of non-protein a Reference (4) substances known. -

Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017
EPA/600/R-17/356 | September 2017 www.epa.gov/homeland-security-research Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 Office of Research and Development Homeland Security Research Program This page left intentionally blank EPA/600/R-17/356 | September 2017 Selected Analytical Methods for Environmental Remediation and Recovery (SAM) 2017 UNITED STATES ENVIRONMENTAL PROTECTION AGENCY Cincinnati, OH 45268 Office of Research and Development Homeland Security Research Program Disclaimer Disclaimer The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development funded and managed the research described here under Contract EP-C-15-012 to CSRA Inc. This document is undergoing review and has not been approved for publication. The contents reflect the views of the contributors and technical work groups and do not necessarily reflect the views of the Agency. Mention of trade names or commercial products in this document or in the methods referenced in this document does not constitute endorsement or recommendation for use. Questions concerning this document or its application should be addressed to: Romy Campisano National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King Drive Cincinnati, OH 45268 (513) 569-7016 [email protected] Kathy Hall National Homeland Security Research Center Office of Research and Development (NG16) U.S. Environmental Protection Agency 26 West Martin Luther King -

NICKEL and the SKIN Absorption, Immunology, Epidemiology, and Metallurgy DERMATOLOGY: CLINICAL & BASIC SCIENCE SERIES Series Editor Howard I
VISIT… NICKEL AND THE SKIN Absorption, Immunology, Epidemiology, and Metallurgy DERMATOLOGY: CLINICAL & BASIC SCIENCE SERIES Series Editor Howard I. Maibach, M.D. Published Titles: Pesticide Dermatoses Homero Penagos, Michael O’Malley, and Howard I. Maibach Hand Eczema, Second Edition Torkil Menné and Howard I. Maibach Dermatologic Botany Javier Avalos and Howard I. Maibach Dry Skin and Moisturizers: Chemistry and Function Marie Loden and Howard I. Maibach Skin Reactions to Drugs Kirsti Kauppinen, Kristiina Alanko, Matti Hannuksela, and Howard I. Maibach Contact Urticaria Syndrome Smita Amin, Arto Lahti, and Howard I. Maibach Bioengineering of the Skin: Skin Surface, Imaging, and Analysis Klaus P. Wilhelm, Peter Elsner, Enzo Berardesca, and Howard I. Maibach Bioengineering of the Skin: Methods and Instrumentation Enzo Berardesca, Peter Elsner, Klaus P. Wilhelm, and Howard I. Maibach Bioengineering of the Skin: Cutaneous Blood Flow and Erythema Enzo Berardesca, Peter Elsner, and Howard I. Maibach Bioengineering of the Skin: Water and the Stratum Corneum Peter Elsner, Enzo Berardesca, and Howard I. Maibach Human Papillomavirus Infections in Dermatovenereology Gerd Gross and Geo von Krogh The Irritant Contact Dermatitis Syndrome Pieter van der Valk, Pieter Coenrads, and Howard I. Maibach Dermatologic Research Techniques Howard I. Maibach Skin Cancer: Mechanisms and Human Relevance Hasan Mukhtar Skin Cancer: Mechanisms and Human Relevance Hasan Mukhtar Protective Gloves for Occupational Use Gunh Mellström, J.E. Walhberg, and Howard I. Maibach Pigmentation and Pigmentary Disorders Norman Levine Nickel and the Skin: Immunology and Toxicology Howard I. Maibach and Torkil Menné Bioengineering of the Skin: Skin Biomechanics Peter Elsner, Enzo Berardesca, Klaus-P. Wilhelm, and Howard I.