Metabolism of Methiocarb and Carbaryl by Rat and Human Livers and Plasma, and Effect on Their PXR, CAR and Pparα Activities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Laboratory Test Report
Aromatic Plant Research Center 230 N 1200 E STE 100 Lehi, UT 84043 www.aromaticplant.org Laboratory Test Report SAMPLE NAME : Cypress CLIENT NAME : ACHS CLIENT LOT # : 36186C Column: ZB5 (60 m length × 0.25 mm inner diameter × 0.25 μm film thickness) Instrument: Shimadzu GCMS-QP2010 Ultra Carrier gas: Helium 80 psi Temperature ramp: 2 degrees Celsius per minute up to 260-degree Celsius Split ratio: 30:1 Sample preparation: 5% w/v solution with Dichloromethane Interpretation on sample: This analysis of Cypress essential oil meets the standard chemical profile of Cupressus sempervirens essential oil. Analyzed by: Dr. Prabodh Satyal Reviewed by: Ambika Poudel Issued Date: 9/25/2020 Copyright © 2020 by Aromatic Plant Research Center (APRC). All rights reserved. The information contained in this document may not be used, published or redistributed, including online, without the prior written consent of APRC. 1 / 4 24,426,457 Chromatogram Injection Volume Lot# Client Name Sample Name Sample Type Analyzed Analyzed by Sample Information www.aromaticplant.org UT Lehi, 84043 100 STE 230N E 1200 Center Aromatic Plant Research 10.856 Copyright © 2020 by Aromatic Plant Research Center (APRC). All rights reserved. Copyright information The All (APRC). rights reserved. this document contained in Center may © 2020by Aromatic Plantnot be used, published Research or 11.74611.898 13.37013.18113.088 12.429 14.335 14.41714.716 15.343 16.015 16.405 16.628 17.032 17.18317.501 18.00417.910 17.812 20.0 18.866 19.30119.646 21.153 21.744 21.445 22.392 : : : 0.30 : : : : ACHS Cy Esse 9/25/2020 7:12:23 AM AM 7:12:23 9/25/2020 Dr. -

4C Pesticide Lists
4C PESTICIDE LISTS Version 4.0 II 4C PESTICIDE LISTS Copyright notice © 2020 4C Services GmbH This document is protected by copyright. It is freely available from the 4C website or upon request. No part of this copyrighted document may be changed or amended. The document may not be duplicated or copied in any form or by any means for commercial purpose without permission of 4C Services. Document Title: 4C Pesticide Lists Version 4.0 Valid from: 01 July 2020 III Content List of Tables ........................................................................................................................ IV Abbreviations ....................................................................................................................... IV 4C PESTICIDE LISTS 1 Introduction ................................................................................................................... 5 2 Selection Criteria Used for the 4C Pesticide Lists .......................................................... 5 3 4C Red List Pesticides: 4C Code of Conduct Requirements and Actions to be Promoted .................................. 6 4 4C Yellow List Pesticides: 4C Code of Conduct Requirements and Actions to be Promoted .................................. 7 © 4C Services GmbH IV List of Tables Table 1: 4C list of unacceptable pesticides ............................................................................ 8 Table 2: 4C red pesticide list ................................................................................................. 9 Table -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

Lists for Pesticide Management
Rainforest Alliance Lists for Pesticide Management Lists of Prohibited and Risk Mitigation Use Pesticides July, 2017 Version 1.3 D.R. © 2017 Red de Agricultura Sostenible, A.C. This document is provided by Red de Agricultura Sostenible, A.C. (also known as Sustainable Agriculture Network) to Rainforest Alliance, Inc. and/or to its successors, under the terms and subject to the limitations set forth in the perpetual, exclusive, non-transferrable license granted by Red de Agricultura Sostenible, A.C. in favor of Rainforest Alliance, Inc., or its successors under the terms and conditions set forth in an agreement between the parties (the “Agreement”), in the understanding that: 1. All content of this document, including, but not limited to text, logos, if any, graphics, photographs, trade names, etc. of Red de Agricultura Sostenible, A.C, is subject to copyright protection in favor of the Red de Agricultura Sostenible, A.C. and third party owners who have duly authorized the inclusion of their work, under the provisions of the Mexican Federal Law on Copyright (Ley Federal del Derecho de Autor) and other related national and / or international laws. The Rainforest Alliance name and trademarks are the sole property of Rainforest Alliance. 2. Rainforest Alliance, Inc., and / or its successors, shall only use the copyrighted material under the terms and conditions of the Agreement. 3. Under no circumstance shall it be understood that a license, of any kind, over this document has been granted to any third party different from Rainforest Alliance, Inc., or its successors. 4. Except for the terms and conditions set forth in the Agreement, under no circumstance shall it be understood that Red de Agricultura Sostenible, A.C. -

Acutely Toxic Chemical List
EPA: Acutely Toxic Chemicals List United States Environmental Protection Agency (EPA) Acutely Toxic Chemical Name EPA Waste Code CAS # Acetaldehyde, chloro- P023 107-20-0 Acetamide, N-(aminothioxomethyl)- P002 591-08-2 Acetamide, 2-fluoro- P057 640-19-7 Acetic acid, fluoro-, sodium salt P058 62-74-8 1-Acetyl-2-thiourea P002 591-08-2 Acrolein P003 107-02-8 Aldicarb P070 116-06-3 Aldicarb sulfone. P203 1646-88-4 Aldrin P004 309-00-2 Allyl alcohol P005 107-18-6 Aluminum phosphide (R,T) P006 20859-73-8 5-(Aminomethyl)-3-isoxazolol P007 2763-96-4 4-Aminopyridine P008 504-24-5 Ammonium picrate (R) P009 131-74-8 Ammonium vanadate P119 7803-55-6 Argentate(1-), bis(cyano-C)-, potassium P099 506-61-6 Arsenic acid H3AsO4 P010 7778-39-4 Arsenic oxide As2 O3 P012 1327-53-3 Arsenic oxide As2O5 P011 1303-28-2 Arsenic pentoxide P011 1303-28-2 Arsenic trioxide P012 1327-53-3 Arsine, diethyl- P038 692-42-2 Arsonous dichloride, phenyl- P036 696-28-6 Aziridine P054 151-56-4 Aziridine, 2-methyl- P067 75-55-8 Barium cyanide P013 542-62-1 Benzenamine, 4-chloro- P024 106-47-8 Benzenamine, 4-nitro- P077 100-01-6 Benzene, (chloromethyl)- P028 100-44-7 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P042 51-43-4 Benzeneethanamine, alpha, alpha-dimethyl- P046 122-09-8 Updated September 2021 T:\Documentation\EHS-Updates\Acute Waste Codes.docx CONTINUED: Liquid Nitrogen Handling and Use United States Environmental Protection Agency (EPA) Acutely Toxic Chemical Name EPA Waste Code CAS # Benzenethiol P014 108-98-5 7-Benzofuranol, 2,3-dihydro-2,2-dimethyl-, methylcarbamate. -
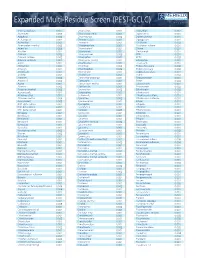
Pesticide Expanded Multi-Residue Screens
www.merieuxnutrisciences.com/us Expanded Multi-Residue Screen (PEST-GCLC) Tel: +1 (312) 938-5151 3-OH-carbofuran 0.001 Chloridazon 0.002 Dioxathion 0.002 Abamectin 0.005 Chlorimuron ethyl 0.001 Diphenamid 0.001 Acephate 0.002 Chlormephos 0.002 Diphenylamine 0.001 Acetamipred 0.002 Chlorobenzilate 0.001 Dipropetryn 0.001 Acetochlor 0.001 Chloroneb 0.001 Disulfoton 0.002 Acibenzolar-s-methyl 0.002 Chloropropilate 0.001 Disulfoton sulfone 0.001 Aclonifen 0.005 Chlorothalonil 0.001 Diuron 0.001 Alachlor 0.001 Chloroxuron 0.001 Dodemorph 0.001 Aldicarb 0.002 Chlorpropham 0.003 EPN 0.001 Aldicarb sulfone 0.002 Chlorpyrifos 0.001 EPTC 0.003 Aldicarb sulfoxide 0.002 Chlorpyrifos-methyl 0.001 Edifenphos 0.001 Aldrin 0.001 Chlorthiamid 0.002 Emamectin 0.001 Allidochlor 0.001 Chlorthion 0.002 Endosulfan alpha 0.002 Ametryn 0.001 Chlorthiophos 0.002 Endosulfan beta 0.003 Aminocarb 0.001 Chlortoluron 0.001 Endosulfan sulfate 0.001 Anilofos 0.001 Chlozolinate 0.001 Endrin 0.002 Aramite I 0.002 Clodinafop-propargyl 0.001 Epoxyconazole 0.001 Aramite II 0.003 Clomazone 0.001 Erbon 0.003 Aspon 0.001 Cloquintocet-methyl 0.001 Esfenvalerate 0.001 Atrazine 0.002 Clothianidin 0.001 Etaconazole 0.002 Atrazine-desethyl 0.002 Coumaphos 0.002 Ethaluralin 0.002 Azaconazole 0.001 Crotoxyphos 0.003 Ethiofencarb 0.001 Azinphos-ethyl 0.002 Cruformate 0.001 Ethiofencarb sulfone 0.001 Azinphos-methyl 0.006 Cyanazine 0.002 Ethiofencarb sulfoxide 0.001 Azoxystrobin 0.003 Cyanofenphos 0.001 Ethion 0.001 BHC alpha isomer 0.001 Cyanophos 0.001 Ethiprole 0.001 BHC beta isomer -

Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 Theinternational Programme on Chemical Safety (IPCS) Was Established in 1980
The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 cation Hazard of Pesticides by and Guidelines to Classi The WHO Recommended Classi The WHO Recommended Classi cation of Pesticides by Hazard and Guidelines to Classi cation 2019 The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification 2019 TheInternational Programme on Chemical Safety (IPCS) was established in 1980. The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals. This publication was developed in the IOMC context. The contents do not necessarily reflect the views or stated policies of individual IOMC Participating Organizations. The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase international coordination in the field of chemical safety. The Participating Organizations are: FAO, ILO, UNDP, UNEP, UNIDO, UNITAR, WHO, World Bank and OECD. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment. WHO recommended classification of pesticides by hazard and guidelines to classification, 2019 edition ISBN 978-92-4-000566-2 (electronic version) ISBN 978-92-4-000567-9 (print version) ISSN 1684-1042 © World Health Organization 2020 Some rights reserved. -

Residues of Carbosulfan and Its Carbofuran Metabolites and 3-Hydroxy-Carbofuran in Oranges1, 2
230 RESIDUES OF CARBOSULFAN AND ITS CARBOFURAN METABOLITES AND 3-HYDROXY-CARBOFURAN IN ORANGES1, 2 MARCOS JOSÉ TREVISAN3, GILBERTO CASADEI DE BAPTISTA4, LUIZ ROBERTO PIMENTEL TREVIZAN4, GERALDO PAPA5 ABSTRACT – The objectives of this study were to evaluate the residues of the insecticide carbosulfan and its carbofuran metabolites and 3- hydroxy-carbofuran in orange compartments (whole fruit, bagasse and juice) and comparison between the residual levels found in fruits with the maximum residue level and the safety interval established by the Brazilian legislation. Two field experiments were carried out, both with the following treatments: a-check; b-one application of 10 g of carbosulfan . 100 L-1 of water; c-one application with twice the rate applied in treatment b; d-four applications with the same rate applied in treatment b. Samples were taken at (-1), zero, 1, 3, 7, 14, 21 and 28 days after the last or unique application. The quantitative determinations were done by gas chromatography technique, using a nitrogen-phosphorus detector. The carbosulfan metabolism to its carbofuran metabolite was rapid (3 days), being both analytes concentrated in the bagasse (peel + flavedo + albedo). However, the metabolism of carbofuran to 3-hydroxy-carbofuran was of low intensity or this metabolite was quickly dissipated. Carbosulfan residues and its metabolites did not penetrate into the fruit, thus not contaminating the juice. The use of the pesticide was adequate, with respect to fruit consumption, in relation to the Brazilian legislation. Index -

Pesticide Residues : Maximum Residue Limits
THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives ICS 67.040 ISBN UNOFFICAL TRANSLATION THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives 50 Phaholyothin Road, Ladyao, Chatuchak, Bangkok 10900 Telephone (662) 561 2277 Fascimile: (662) 561 3357 www.acfs.go.th Published in the Royal Gazette, Announcement and General Publication Volume 131, Special Section 32ง (Ngo), Dated 13 February B.E. 2557 (2014) (2) Technical Committee on the Elaboration of the Thai Agricultural Standard on Maximum Residue Limits for Pesticide 1. Mrs. Manthana Milne Chairperson Department of Agriculture 2. Mrs. Thanida Harintharanon Member Department of Livestock Development 3. Mrs. Kanokporn Atisook Member Department of Medical Sciences, Ministry of Public Health 4. Mrs. Chuensuke Methakulawat Member Office of the Consumer Protection Board, The Prime Minister’s Office 5. Ms. Warunee Sensupa Member Food and Drug Administration, Ministry of Public Health 6. Mr. Thammanoon Kaewkhongkha Member Office of Agricultural Regulation, Department of Agriculture 7. Mr. Pisan Pongsapitch Member National Bureau of Agricultural Commodity and Food Standards 8. Ms. Wipa Thangnipon Member Office of Agricultural Production Science Research and Development, Department of Agriculture 9. Ms. Pojjanee Paniangvait Member Board of Trade of Thailand 10. Mr. Charoen Kaowsuksai Member Food Processing Industry Club, Federation of Thai Industries 11. Ms. Natchaya Chumsawat Member Thai Agro Business Association 12. Mr. Sinchai Swasdichai Member Thai Crop Protection Association 13. Mrs. Nuansri Tayaputch Member Expert on Method of Analysis 14. -

List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde