Spectrophotometric Determination of Carbosulfan in Environmental Samples
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Code Chemical P026 1-(O-Chlorophenyl)Thiourea P081 1
Code Chemical P026 1-(o-Chlorophenyl)thiourea P081 1,2,3-Propanetriol, trinitrate (R) P042 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P067 1,2-Propylenimine P185 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)- carbonyl]oxime 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a,-hexahydro-, P004 (1alpha,4alpha, 4abeta,5alpha,8alpha,8abeta)- 1,4,5,8-Dimethanonaphthalene, 1,2,3,4,10,10-hexa- chloro-1,4,4a,5,8,8a-hexahydro-, P060 (1alpha,4alpha, 4abeta,5beta,8beta,8abeta)- P002 1-Acetyl-2-thiourea P048 2,4-Dinitrophenol P051 2,7:3,6-Dimethanonaphth [2,3-b]oxirene, 3,4,5,6,9,9 -hexachloro-1a,2,2a,3,6,6a,7,7a- octahydro-, (1aalpha,2beta,2abeta,3alpha,6alpha,6abeta,7 beta, 7aalpha)-, & metabolites 2,7:3,6-Dimethanonaphth[2,3-b]oxirene, 3,4,5,6,9,9- hexachloro-1a,2,2a,3,6,6a,7,7a- P037 octahydro-, (1aalpha,2beta,2aalpha,3beta,6beta,6aalpha,7 beta, 7aalpha)- P045 2-Butanone, 3,3-dimethyl-1-(methylthio)-, O-[methylamino)carbonyl] oxime P034 2-Cyclohexyl-4,6-dinitrophenol 2H-1-Benzopyran-2-one, 4-hydroxy-3-(3-oxo-1- phenylbutyl)-, & salts, when present at P001 concentrations greater than 0.3% P069 2-Methyllactonitrile P017 2-Propanone, 1-bromo- P005 2-Propen-1-ol P003 2-Propenal P102 2-Propyn-1-ol P007 3(2H)-Isoxazolone, 5-(aminomethyl)- P027 3-Chloropropionitrile P047 4,6-Dinitro-o-cresol, & salts P059 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro- 3a,4,7,7a-tetrahydro- P008 4-Aminopyridine P008 4-Pyridinamine P007 5-(Aminomethyl)-3-isoxazolol 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- -

Metabolism of Methiocarb and Carbaryl by Rat and Human Livers and Plasma, and Effect on Their PXR, CAR and Pparα Activities
The Journal of Toxicological Sciences (J. Toxicol. Sci.) 677 Vol.41, No.5, 677-691, 2016 Original Article Metabolism of methiocarb and carbaryl by rat and human livers and plasma, and effect on their PXR, CAR and PPARα activities Chieri Fujino1, Yuki Tamura2, Satoko Tange1, Hiroyuki Nakajima3,4, Seigo Sanoh1, Yoko Watanabe2, Naoto Uramaru2, Hiroyuki Kojima5, Kouichi Yoshinari3,4, Shigeru Ohta1 and Shigeyuki Kitamura2 1Graduate School of Biomedical and Health Sciences, Hiroshima University, Kasumi 1-2-3, Minami-ku, Hiroshima 734-8553, Japan 2Nihon Pharmaceutical University, Komuro 10281, Ina-machi, Kitaadachi-gun, Saitama 362-0806, Japan 3Graduate School of Pharmaceutical Sciences, Tohoku University, 6-3 Aramaki-Aoba, Aoba-ku, Sendai 980-8578, Japan 4School of Pharmaceutical Sciences, University of Shizuoka, 52-1 Yada, Suruga-ku, Shizuoka 422-8526, Japan 5Hokkaido Institute of Public Health, Kita-19, Nishi-12, Kita-ku, Sapporo 060-0819, Japan (Received April 14, 2016; Accepted August 2, 2016) ABSTRACT — The oxidative, reductive, and hydrolytic metabolism of methiocarb and the hydrolyt- ic metabolism of carbaryl by liver microsomes and plasma of rats or humans were examined. The effects of the metabolism of methiocarb and carbaryl on their nuclear receptor activities were also examined. When methiocarb was incubated with rat liver microsomes in the presence of NADPH, methiocarb sul- foxide, and a novel metabolite, methiocarb sulfone were detected. Methiocarb sulfoxide was oxidized to the sulfone by liver microsomes and reduced back to methiocarb by liver cytosol. Thus, the intercon- version between methiocarb and the sulfoxide was found to be a new metabolic pathway for methiocarb by liver microsomes. -

Acutely Toxic Chemical List
EPA: Acutely Toxic Chemicals List United States Environmental Protection Agency (EPA) Acutely Toxic Chemical Name EPA Waste Code CAS # Acetaldehyde, chloro- P023 107-20-0 Acetamide, N-(aminothioxomethyl)- P002 591-08-2 Acetamide, 2-fluoro- P057 640-19-7 Acetic acid, fluoro-, sodium salt P058 62-74-8 1-Acetyl-2-thiourea P002 591-08-2 Acrolein P003 107-02-8 Aldicarb P070 116-06-3 Aldicarb sulfone. P203 1646-88-4 Aldrin P004 309-00-2 Allyl alcohol P005 107-18-6 Aluminum phosphide (R,T) P006 20859-73-8 5-(Aminomethyl)-3-isoxazolol P007 2763-96-4 4-Aminopyridine P008 504-24-5 Ammonium picrate (R) P009 131-74-8 Ammonium vanadate P119 7803-55-6 Argentate(1-), bis(cyano-C)-, potassium P099 506-61-6 Arsenic acid H3AsO4 P010 7778-39-4 Arsenic oxide As2 O3 P012 1327-53-3 Arsenic oxide As2O5 P011 1303-28-2 Arsenic pentoxide P011 1303-28-2 Arsenic trioxide P012 1327-53-3 Arsine, diethyl- P038 692-42-2 Arsonous dichloride, phenyl- P036 696-28-6 Aziridine P054 151-56-4 Aziridine, 2-methyl- P067 75-55-8 Barium cyanide P013 542-62-1 Benzenamine, 4-chloro- P024 106-47-8 Benzenamine, 4-nitro- P077 100-01-6 Benzene, (chloromethyl)- P028 100-44-7 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)- P042 51-43-4 Benzeneethanamine, alpha, alpha-dimethyl- P046 122-09-8 Updated September 2021 T:\Documentation\EHS-Updates\Acute Waste Codes.docx CONTINUED: Liquid Nitrogen Handling and Use United States Environmental Protection Agency (EPA) Acutely Toxic Chemical Name EPA Waste Code CAS # Benzenethiol P014 108-98-5 7-Benzofuranol, 2,3-dihydro-2,2-dimethyl-, methylcarbamate. -

Residues of Carbosulfan and Its Carbofuran Metabolites and 3-Hydroxy-Carbofuran in Oranges1, 2
230 RESIDUES OF CARBOSULFAN AND ITS CARBOFURAN METABOLITES AND 3-HYDROXY-CARBOFURAN IN ORANGES1, 2 MARCOS JOSÉ TREVISAN3, GILBERTO CASADEI DE BAPTISTA4, LUIZ ROBERTO PIMENTEL TREVIZAN4, GERALDO PAPA5 ABSTRACT – The objectives of this study were to evaluate the residues of the insecticide carbosulfan and its carbofuran metabolites and 3- hydroxy-carbofuran in orange compartments (whole fruit, bagasse and juice) and comparison between the residual levels found in fruits with the maximum residue level and the safety interval established by the Brazilian legislation. Two field experiments were carried out, both with the following treatments: a-check; b-one application of 10 g of carbosulfan . 100 L-1 of water; c-one application with twice the rate applied in treatment b; d-four applications with the same rate applied in treatment b. Samples were taken at (-1), zero, 1, 3, 7, 14, 21 and 28 days after the last or unique application. The quantitative determinations were done by gas chromatography technique, using a nitrogen-phosphorus detector. The carbosulfan metabolism to its carbofuran metabolite was rapid (3 days), being both analytes concentrated in the bagasse (peel + flavedo + albedo). However, the metabolism of carbofuran to 3-hydroxy-carbofuran was of low intensity or this metabolite was quickly dissipated. Carbosulfan residues and its metabolites did not penetrate into the fruit, thus not contaminating the juice. The use of the pesticide was adequate, with respect to fruit consumption, in relation to the Brazilian legislation. Index -

Pesticide Residues : Maximum Residue Limits
THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives ICS 67.040 ISBN UNOFFICAL TRANSLATION THAI AGRICULTURAL STANDARD TAS 9002-2013 PESTICIDE RESIDUES : MAXIMUM RESIDUE LIMITS National Bureau of Agricultural Commodity and Food Standards Ministry of Agriculture and Cooperatives 50 Phaholyothin Road, Ladyao, Chatuchak, Bangkok 10900 Telephone (662) 561 2277 Fascimile: (662) 561 3357 www.acfs.go.th Published in the Royal Gazette, Announcement and General Publication Volume 131, Special Section 32ง (Ngo), Dated 13 February B.E. 2557 (2014) (2) Technical Committee on the Elaboration of the Thai Agricultural Standard on Maximum Residue Limits for Pesticide 1. Mrs. Manthana Milne Chairperson Department of Agriculture 2. Mrs. Thanida Harintharanon Member Department of Livestock Development 3. Mrs. Kanokporn Atisook Member Department of Medical Sciences, Ministry of Public Health 4. Mrs. Chuensuke Methakulawat Member Office of the Consumer Protection Board, The Prime Minister’s Office 5. Ms. Warunee Sensupa Member Food and Drug Administration, Ministry of Public Health 6. Mr. Thammanoon Kaewkhongkha Member Office of Agricultural Regulation, Department of Agriculture 7. Mr. Pisan Pongsapitch Member National Bureau of Agricultural Commodity and Food Standards 8. Ms. Wipa Thangnipon Member Office of Agricultural Production Science Research and Development, Department of Agriculture 9. Ms. Pojjanee Paniangvait Member Board of Trade of Thailand 10. Mr. Charoen Kaowsuksai Member Food Processing Industry Club, Federation of Thai Industries 11. Ms. Natchaya Chumsawat Member Thai Agro Business Association 12. Mr. Sinchai Swasdichai Member Thai Crop Protection Association 13. Mrs. Nuansri Tayaputch Member Expert on Method of Analysis 14. -
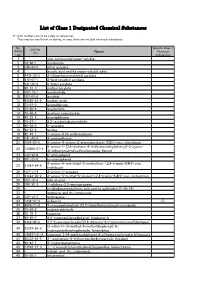
List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde -

Electrochemical Biosensors Based on Acetylcholinesterase and Butyrylcholinesterase
Int. J. Electrochem. Sci., 11 (2016) 7440 – 7452, doi: 10.20964/2016.09.16 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Short Review Electrochemical Biosensors based on Acetylcholinesterase and Butyrylcholinesterase. A Review Miroslav Pohanka Faculty of Military Health Sciences, University of Defense, Trebesska 1575, Hradec Kralove, Czech Republic; Department of Geology and Pedology, Mendel University in Brno, Czech Republic E-mail: [email protected] Received: 8 May 2016 / Accepted: 23 June 2016 / Published: 7 August 2016 Two cholinesterases are known: acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). The both enzymes are presented in the body under physiological conditions and the both enzymes have broad use in pharmacology, toxicology and analyses. In the current paper, construction of electrochemical biosensors, a specific phenomenon in analytical chemistry, is reviewed. Development of voltammetric and potentiometric methods using standard electrodes, nanostructured materials, micro and nanoparticles is described here commonly with depicting of reaction principles and selection of substrates for the cholinesterases. Survey of actual literature is also provided within the article and disparate analytes used for the biosensors testing are introduced. Keywords: Acetylcholinesterase; biosensor; butyrylcholinesterase; conductometry; nerve agent; pesticide; potentiometry; voltammetry 1. INTRODUCTION While AChE (EC 3.1.1.7) is an enzyme with broad interest from pharmacologist developing new drugs, BChE (EC 3.1.1.8) has lower importance because of not clear biological function [1,2]. BChE is expressed by livers and secerned to blood plasma where can be used as a liver function test [2]. Role of AChE is well known because it is a part of cholinergic nervous system where it terminates excitation by hydrolysis of a neurotransmitter acetylcholine in nerve junctions, neuro-muscular junctions, blood and elsewhere [3-5]. -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -
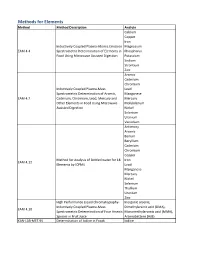
Method Description
Methods for Elements Method Method Description Analyte Calcium Copper Iron Inductively Coupled Plasma-Atomic Emission Magnesium EAM 4.4 Spectrometric Determination of Elements in Phosphorus Food Using Microwave Assisted Digestion Potassium Sodium Strontium Zinc Arsenic Cadmium Chromium Inductively Coupled Plasma-Mass Lead Spectrometric Determination of Arsenic, Manganese EAM 4.7 Cadmium, Chromium, Lead, Mercury and Mercury Other Elements in Food Using Microwave Molybdenum Assisted Digestion Nickel Selenium Uranium Vanadium Antimony Arsenic Barium Beryllium Cadmium Chromium Copper Method for Analysis of Bottled water for 18 Iron EAM 4.12 Elements by ICPMS Lead Manganese Mercury Nickel Selenium Thallium Uranium Zinc High Performance Liquid Chromatography- Inorganic arsenic, Inductively Coupled Plasma-Mass Dimethylarsinic acid (DMA), EAM 4.10 Spectrometric Determination of Four Arsenic Monomethylarsonic acid (MMA), Species in Fruit Juice Arsenobetaine (AsB) KAN-LAB-MET.95 Determination of Iodine in Foods Iodine Methods for Radionuclides Method Method Description Analyte Determination of Strontium-90 in Foods by WEAC.RN.METHOD.2.0 Strontium-90 Internal Gas-Flow Proportional Counting Americium-241 Cesium-134 Cesium-137 Determination of Gamma-Ray Emitting Cobalt-60 WEAC.RN.METHOD.3.0 Radionuclides in Foods by High-Purity Potassium-40 Germanium Spectrometry Radium-226 Ruthenium-103 Ruthenium-106 Thorium-232 Methods for Pesticides/Industrial Chemicals Method Method Description Analyte Extraction Method: Analysis of Pesticides KAN-LAB-PES.53 and -

EURL-SRM - Analytical Observations Report Concerning the Following…
EURL-SRM - Analytical Observations Report concerning the following… o Compound(s): Carbofuran, Benfuracarb, Carbosulfan, Furathiocarb, 3-Hydroxy-Carbofuran o Commodities: Various commodities of plant origin o Method(s): QuEChERS followed by a hydrolysis step o Instrumentation: LC-MS/MS Analysis of Carbofuran (sum) by applying hydrolysis on QuEChERS extracts Reported by: EURL-SRM Version 1 (last update: 20.04.2016) Background information / Initial Observations: Carbofuran (CF) is an insecticide, nematicide and acaricide of the carbamate group. Carbosulfan (CS), furathiocarb (FT) and benfuracarb (BF) are pro-pesticides that degrade to CF, which is the active substance. To account for the high acute neurotoxicity of CF very low toxicological reference values were allocated in 2009 (ARfD 0.00015 mg/kg bw)1 [2]. The metabolite 3-OH-carbofuran (3-OH-CF) was considered exhibiting similar toxicity as the parent. Originally CS, BF and FT were regulated separately from CF and its metabolite 3-OH-CF. The separate MRLs of CS and BF were, however, difficult to enforce as these two compounds are highly susceptible to degradation during extraction and extract storage, especially where the pH of the matrix is low. For this reason, the QuEChERS protocol (EN-15662) foresees not acidifying the extracts after cleanup by dispersive SPE with PSA, to minimize losses between extraction and measurement. But even then, further losses are expected during sample milling, sample extraction (especially in acidic commodities prior to buffering) or storage of homogenates, especially if pH is low and temperatures high. In 2012 the MRLs of CF, BF, CS and FT for most products were lowered to their respective consensus LOQ at that time2.