A Contribution on the Neglected Milliped Genus <I>Apheloria</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diversity of Millipedes Along the Northern Western Ghats
Journal of Entomology and Zoology Studies 2014; 2 (4): 254-257 ISSN 2320-7078 Diversity of millipedes along the Northern JEZS 2014; 2 (4): 254-257 © 2014 JEZS Western Ghats, Rajgurunagar (MS), India Received: 14-07-2014 Accepted: 28-07-2014 (Arthropod: Diplopod) C. R. Choudhari C. R. Choudhari, Y.K. Dumbare and S.V. Theurkar Department of Zoology, Hutatma Rajguru Mahavidyalaya, ABSTRACT Rajgurunagar, University of Pune, The different vegetation type was used to identify the oligarchy among millipede species and establish India P.O. Box 410505 that millipedes in different vegetation types are dominated by limited set of species. In the present Y.K. Dumbare research elucidates the diversity of millipede rich in part of Northern Western Ghats of Rajgurunagar Department of Zoology, Hutatma (MS), India. A total four millipedes, Harpaphe haydeniana, Narceus americanus, Oxidus gracilis, Rajguru Mahavidyalaya, Trigoniulus corallines taxa belonging to order Polydesmida and Spirobolida; 4 families belongs to Rajgurunagar, University of Pune, Xystodesmidae, Spirobolidae, Paradoxosomatidae and Trigoniulidae and also of 4 genera were India P.O. Box 410505 recorded from the tropical or agricultural landscape of Northern Western Ghats. There was Harpaphe haydeniana correlated to the each species of millipede which were found in Northern Western Ghats S.V. Theurkar region of Rajgurunagar. At the time of diversity study, Trigoniulus corallines were observed more than Senior Research Fellowship, other millipede species, which supports the environmental determinism condition. Narceus americanus Department of Zoology, Hutatma was single time occurred in the agricultural vegetation landscape due to the geographical location and Rajguru Mahavidyalaya, habitat differences. Rajgurunagar, University of Pune, India Keywords: Diplopod, Northern Western Ghats, millipede diversity, Narceus americanus, Trigoniulus corallines 1. -

(Polydesmida: Leptodesmidea) in the West Indies: Proposal of Hoffmanorhacus N
Zootaxa 3626 (4): 477–498 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3626.4.4 http://zoobank.org/urn:lsid:zoobank.org:pub:24763A0D-5B5F-4DDB-9698-5D608738FE4D The milliped family Platyrhacidae (Polydesmida: Leptodesmidea) in the West Indies: Proposal of Hoffmanorhacus n. gen.; description and illustrations of males of Proaspis aitia Loomis, 1941; redescription of Nannorrhacus luciae (Pocock, 1894); hypotheses on origins and affinities; and an updated New World familial distribution ROWLAND M. SHELLEY1 & DANIELA MARTINEZ-TORRES2 1Research Laboratory, North Carolina State Museum of Natural Sciences, MSC #1626, Raleigh, NC 27699-1626 USA. E-mail: [email protected] 2Instituto de Ciencias Naturales,Universidad Nacional de Colombia, Edificio 425, Oficina 105, Bogotá, Colombia. E-mail: [email protected] Abstract In the New World, the milliped family Platyrhacidae (Polydesmida) is known or projected for Central America south of southeastern Nicaragua and the northern ¼ of South America, with disjunct, insular populations on Hispaniola (Haiti), Guadeloupe (Basse-Terre), and St. Lucia. Male near-topotypes enable redescription of Proaspis aitia Loomis, 1941, possibly endemic to the western end of the southern Haitian peninsula. The tibiotarsus of its biramous gonopodal telopodite bends strongly laterad, and the medially directed solenomere arises at midlength proximal to the bend. With a uniramous telopodite, P. sahlii Jeekel, 1980, on Guadeloupe, is not congeneric, and Hoffmanorhacus, n. gen., is erected to accommodate it. Nannorrhacus luciae (Pocock, 1894), on St. Lucia, is redescribed; also with a biramous telopodite, its tibiotarsus arises distad and diverges from the coaxial solenomere. -

Gonopod Mechanics in Centrobolus Cook (Spirobolida: Trigoniulidae) II
Journal of Entomology and Zoology Studies 2016; 4(2): 152-154 E-ISSN: 2320-7078 P-ISSN: 2349-6800 JEZS 2016; 4(2): 152-154 Gonopod mechanics in Centrobolus Cook © 2016 JEZS (Spirobolida: Trigoniulidae) II. Images Received: 06-01-2016 Accepted: 08-02-2016 Mark Ian Cooper Mark Ian Cooper A) Department of Biological Sciences, Private Bag X3, Abstract University of Cape Town, Gonopod mechanics were described for four species of millipedes in the genus Centrobolus and are now Rondebosch 7701, South Africa. figured using scanning electron microscopy (SEM) with the aim to show the mechanism of sperm B) Electron Microscope Unit & Structural Biology Research competition. Structures of sperm displacement include projections on a moveable telopodite and tips on a Unit, University of Cape Town, distal process (opisthomerite). Three significant contact zones between the male and female genitalia South Africa. were recognized: (1) distal telopodite of the coleopod and the vulva, (2) phallopod and the bursa, (3) sternite and legs of the female. Keywords: coleopods, diplopod, gonopods, phallopods 1. Introduction The dual function of millipede male genitalia in sperm displacement and transfer were predicted from the combined examination of the ultrastructures of the male and female genitalia [1-3]. Genitalic structures function do not only in sperm transfer during the time of copulation, but that they perform copulatory courtship through movements and interactions with the female genitalia [4-5]. These 'functional luxuries' can induce cryptic female choice by stimulating structures on the female genitalia while facilitating rival-sperm displacement and sperm transfer. Genitalic complexity is probably underestimated in many species because they have only been studied in the retracted or relaxed state [4]. -

A New Genus of the Millipede Tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean Region
European Journal of Taxonomy 70: 1-12 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2013.70 www.europeanjournaloftaxonomy.eu 2013 · Lazányi E. & Vagalinski B. This work is licensed under a Creative Commons Attribution 3.0 License. Research article urn:lsid:zoobank.org:pub:5E23F454-2A68-42F6-86FB-7D9952B2FE7B A new genus of the millipede tribe Brachyiulini (Diplopoda: Julida: Julidae) from the Aegean region Eszter LAZÁNYI1,4 & Boyan VAGALINSKI2,3,5 1 Corresponding author: Department of Zoology, Hungarian Natural History Museum, Baross u. 13, H-1088 Budapest, Hungary. E-mail: [email protected] 2 Faculty of Biology, Sofia University, 8 Dragan Tsankov Blvd., 1164 Sofia, Bulgaria. E-mail: [email protected] 3 Institute of Biodiversity and Ecosystem Research, Bulgarian Academy of Sciences, 2 Yurii Gagarin Street, 1113, Sofia, Bulgaria. 4 urn:lsid:zoobank.org:author:02DB48F1-624C-4435-AF85-FA87168CD85A 5 urn:lsid:zoobank.org:author:973B8725-039E-4F29-8D73-96A7F52CF934 Abstract. A new genus of the julid tribe Brachyiulini, Enghophyllum gen. nov., is described, comprising two species from Greece. The type-species, E. naxium (Verhoeff, 1901) comb. nov. (ex Megaphyllum Verhoeff, 1894), appears to be rather widespread in the Aegean: it is known from Antiparos Island and Naxos Island (the type locality), both in the Cyclades, as well as East Mavri Islet, Dodecanese Archipelago (new record). The vulva of E. naxium is described for the first time. In addition,E. sifnium gen. et sp. nov. is described based on a single adult male from Sifnos Island, Cyclades. The new genus is distinct from other genera of the Brachyiulini mainly by its peculiar gonopod structure, apparently disjunct and at least mostly apomorphous: (1) promeres broad, shield-like, in situ protruding mostly posteriad, completely covering the opisthomeres and gonopodal sinus; (2) transverse muscles and coxal apodemes of promere fully reduced; (3) opisthomere with three differentiated processes, i.e., lateral, basal posterior and apical posterior; (4) solenomere rather simple, tubular. -

Mnohonôžky (Diplopoda) Mesta Banská Bystrica (Stredné Slovensko)
Folia faunistica Slovaca 17 (3) 2012: 291–296 www.ffs.sk Mnohonôžky (DiplopoDa) Mesta Banská Bystrica (streDné slovensko) Slavomír Stašiov, Gabriela Fridrichová, Šimon Kertys, Lucia Miňová, Andrea Uhlíková & Peter Urblík Katedra biológie a všeobecnej ekológie, Fakulta ekológie a environmentalistiky, Technická univerzita vo Zvolene, T. G. Masaryka 24, 960 53 Zvolen, Slovakia [[email protected]] abstract: The paper deals with the results of research focused on the species structure of millipede fauna on 6 sites in the town Banská Bystrica (Central Slovakia).Julus curvicornis Millipedes were captured by pitfall trapping in 2011. In total, 519- individuals from 11 species were recorded in the researched area. The records of J. curvicornis Verhoeff, 1899 is interesting because the species was re corded in the urban environment only the second time generally. We can be assume that is able to colonize wider range of habitats than was previouslykey words: believed. Banská Bystrica, Diplopoda, Slovakia, urban. úvoD - uvedených sídiel, alebo z ich okolia. Mnohonôžky v- - extraviláne Bratislavy študovali Mišík et al. (1974), Faunistický výskum sa u nás už tradične sústre - Krumpál (1993), Mock & Janský (2000), Holeco ďuje predovšetkým na chránené časti prírody, pri - - vá et al. (2005), Stašiov (2005; nepubl.). Sporadic čom urbánne prostredie je zväčša mimo záujmu - ké údaje o mnohonôžkach z iných miest a obcí Slo väčšiny zoológov. Platí to aj o výskume mnohonô venska prinášajú tiež práce Mock & Janský (2000),- žok. Najstaršie údaje o výskyte mnohonôžok v in- Mock (2001a, 2001b, 2004, 2006), Stašiov (2004, travilánoch z územia Slovenska poskytuje práca 2009), Hazuchová et al. (2008, 2009), Palkovičo Szakmáry (1891). Obsahuje záznamy nálezov via vá & Mock (2008), Droběnová & Mock (2009) a cerých druhov mnohonôžok v Banskej Štiavnici, - Sklených Tepliciach a vo Vyhniach. -

Centre International De Myriapodologie
N° 28, 1994 BULLETIN DU ISSN 1161-2398 CENTRE INTERNATIONAL DE MYRIAPODOLOGIE [Mus6umNationald'HistoireNaturelle,Laboratoire de Zoologie-Arthropodes, 61 rue de Buffon, F-75231 ParisCedex05] LISTE DES TRAVAUX PARUS ET SOUS-PRESSE LIST OF WORKS PUBLISHED OR IN PRESS MYRIAPODA & ONYCHOPHORA ANNUAIRE MONDIAL DES MYRIAPODOLOGISTES WORLD DIRECTORY OF THE MYRIAPODOLOGISTS PUBLICATION ET LISIES REPE&TORIEES PANS LA BASE PASCAL DE L' INIST 1995 N° 28, 1994 BULLETIN DU ISSN 1161-2398 CENTRE INTERNATIONAL DE MYRIAPODOLOGIE [Museum National d'Histoire N aturelle, Laboratoire de Zoologie-Arthropodes, 61 rue de Buffon, F-7 5231 Paris Cedex 05] LISTE DES TRAVAUX PARUS ET SOUS-PRESSE LIST OF WORKS PUBLISHED OR IN PRESS MYRIAPODA & ONYCHOPHORA ANNUAIRE MONDIAL DES MYRIAPODOLOGISTES WORLD DIRECTORY OF THE MYRIAPODOLOGISTS PUBLICATION ET LISTES REPERTORIEES DANS LA BASE PASCAL DE L' INIST 1995 SOMMAIRE CONTENTS ZUSAMMENFASSUNG Pages Seite lOth INTERNATIONAL CONGRESS OF MYRIAPODOLOGY .................................. 1 9th CONGRES INTERNATIONAL DE MYRIAPODOLOGIE.................................................... 1 Contacter le Secretariat permanent par E-M AIL & FA X............................................................ 1 The Proceedings of the 9th International Congress of Myriapodology...................... 2 MILLEPATTIA, sommaire .du prochain bulletin....................................................................... 2 Obituary: Colin Peter FAIRHURST (1942-1994) ............................................................. 3 BULLETIN of the -

Apheloria Polychroma, a New Species of Millipede from the Cumberland Mountains (Polydesmida: Xystodesmidae) PAUL E. MAREK1*
Apheloria polychroma, a new species of millipede from the Cumberland Mountains (Polydesmida: Xystodesmidae) PAUL E. MAREK1*, JACKSON C. MEANS1, DEREK A. HENNEN1 1Virginia Polytechnic Institute and State University, Department of Entomology, Blacksburg, Virginia 24061, U.S.A. *Corresponding author, email: [email protected] Abstract Millipedes of the genus Apheloria occur in temperate broadleaf forests throughout eastern North America and west of the Mississippi River in the Ozark and Ouachita Mountains. Chemically defended with toxins made up of cyanide and benzaldehyde, the genus is part of a community of xystodesmid millipedes that compose several Müllerian mimicry rings in the Appalachian Mountains. We describe a model species of these mimicry rings, Apheloria polychroma n. sp., one of the most variable in coloration of all species of Diplopoda with more than six color morphs, each associated with a separate mimicry ring. Keywords: aposematic, Appalachian, Myriapoda, taxonomy, systematics Introduction Millipedes in the family Xystodesmidae are most diverse in the Appalachian Mountains where about half of the family’s species occur. In the New World, the family is distributed throughout eastern and western North America and south to El Salvador (Marek et al. 2014, Marek et al. 2017). Xystodesmidae occur in the Old World in the Mediterranean, the Russian Far East, Japan, western and eastern China, Taiwan and Vietnam. Taxa include species that are bioluminescent (genus Motyxia) and highly gregarious (genera Parafontaria and Pleuroloma); some form Müllerian mimicry rings. Despite their fascinating biology and critical ecological function as native decomposers in broadleaf deciduous forests in the U.S., their alpha-taxonomy is antiquated, and scores of new species remain undescribed. -
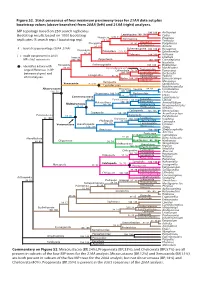
Figure S2. Strict Consensus of Four Maximum Parsimony Trees for 21AA Data Set Plus Bootstrap Values (Above Branches) from 20AA (Left) and 21AA (Right) Analyses
Figure S2. Strict consensus of four maximum parsimony trees for 21AA data set plus bootstrap values (above branches) from 20AA (left) and 21AA (right) analyses. MP topology based on 250 search replicates. 100 100 Antheraea Bootstrap results based on 1000 bootstrap Lepidoptera 100 100 Cydia Neoptera [23] 44 Dermaptera Prodoxus replicates (5 search reps / bootstrap rep). 68 66 Forficula Blattodea Pterygota 92 96 Periplaneta Orthoptera 96 97 Acheta # : bootstrap percentage (20AA 21AA) Ephemeroptera Dicondylia 100 100 Hexagenia Paleoptera [37] 52 Ephemerella 98 98 Odonata 100 100 Ischnura [ ] : node not present in 20AA Insecta Libellula MP strict consensus 100 100 Zygentoma 100 100 Ctenolepisma Nicoletia Archaeognatha : Identifies 6 taxa with Hexapoda 100 100 Pedetontus 89 86 Machiloides Entomobryomorpha Tomoceridae large differences in BP Collembola 70 76 Tomocerus between degen1 and 100 100 Entomobryidae Orchesella Entognatha 82 82 Poduridae Podura 20AA analyses. Diplura 100 100 Campodeidae Japygidae Eumesocampa Remipedia Metajapyx Xenocarida [31] 51 Speleonectes Cephalocarida Hutchinsoniella Altocrustacea Thoracica Sessilia 88 83 Semibalanus [14] 31 100 100 Chthamalus Thecostraca 100 100 Pedunculata Lepas Communostraca Rhizocephala Loxothylacus 96 89 Eumalacostraca 84 85 Eucarida Libinia Malacostraca 100 100 Peracarida Armadillidium Multicrustacea 100 100 Hoplocarida 69 84 Neogonodactylus Phyllocarida Nebalia Cyclopoida 100 100 Mesocyclops Copepoda 100 100 Acanthocyclops Pancrustacea 65 82 Calanoida Eurytemora 96 100 Diplostraca 100 100 -

A Mountain of Millipedes IX: Species of the Family
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: European Journal of Taxonomy Jahr/Year: 2020 Band/Volume: 0675 Autor(en)/Author(s): Olsen Sissel Anna, Rosenmejer Trine, Enghoff Henrik Artikel/Article: A mountain of millipedes IX: Species of the family Gomphodesmidae from the Udzungwa Mountains, Tanzania (Diplopoda, Polydesmida) 1-35 European Journal of Taxonomy 675: 1–35 ISSN 2118-9773 https://doi.org/10.5852/ejt.2020.675 www.europeanjournaloftaxonomy.eu 2020 · Olsen S.A. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:88FFA67B-C2DE-43C6-ACB1-44EDCF119EBA A mountain of millipedes IX: Species of the family Gomphodesmidae from the Udzungwa Mountains, Tanzania (Diplopoda, Polydesmida) Sissel Anna OLSEN 1, Trine ROSENMEJER 2 & Henrik ENGHOFF 3,* 1,2,3 Natural History Museum of Denmark, University of Copenhagen, Universitetsparken 15, DK-2100 København Ø, Denmark * Corresponding author: [email protected] 1 Email: [email protected] 2 Email: [email protected] 1 urn:lsid:zoobank.org:author:4E58B96D-3B55-46AF-90A8-90C8EAF6570C 2 urn:lsid:zoobank.org:author:F9E1E73B-E6D8-46D7-B470-05E91142C98F 3 urn:lsid:zoobank.org:author:FB09A817-000D-43C3-BCC4-2BC1E5373635 Abstract. A new genus and six new species of the family Gomphodesmidae from the Udzungwa Mts are described, including Pogoro alopias Rosenmejer & Enghoff sp. nov., Pogoro siren Rosenmejer & Enghoff sp. nov., Pogoropsis prolixopes Rosenmejer & Enghoff gen. et sp. nov., Emphysemastix frampt Olsen & Enghoff sp. nov., Agrophogonus hamulus Olsen & Enghoff sp. nov., and Agrophogonus pusillokiellandi Olsen & Enghoff sp. -

Order CALLIPODIDA Manual Versión Española
Revista IDE@ - SEA, nº 25B (30-06-2015): 1–12. ISSN 2386-7183 1 Ibero Diversidad Entomológica @ccesible www.sea-entomologia.org/IDE@ Class: Diplopoda Order CALLIPODIDA Manual Versión española CLASS DIPLOPODA Order Callipodida Jörg Spelda Bavarian State Collection of Zoology Münchhausenstraße 21, 81247 Munich, Germany [email protected] 1. Brief characterization of the group and main diagnostic characters 1.1. Morphology The members of the order Callipodida are best recognized by their putative apomorphies: a divided hypoproct, divided anal valves, long extrusible tubular vulvae, and, as in all other helminthomorph milli- pede orders, a characteristic conformation of the male gonopods. As in Polydesmida, only the first leg pair of the 7th body ring is transformed into gonopods, which are retracted inside the body. Body rings are open ventrally and are not fused with the sternites, leaving the coxae of the legs free. Legs in the anterior half of the body carry coxal pouches. The small collum does not overlap the head. Callipodida are of uniformly cylindrical external appearance. The number of body rings is only sometimes fixed in species and usually exceeds 40. There are nine antennomeres, as the 2nd antennomere of other Diplopoda is subdivided (= antennomere 2 and 3 in Callipodida). The general struc- ture of the gnathochilarium is shared with the Chordeumatida and Polydesmida. Callipodida are said to be characterised by longitudinal crests, which gives the order the common name “crested millipedes”. Although crest are present in most species, some genera (e.g. Schizopetalum) lack a crest, while some Spirostreptida ( e.g. in Cambalopsidae, ‘Trachystreptini’) and some Julida (e.g. -

Biodiversity from Caves and Other Subterranean Habitats of Georgia, USA
Kirk S. Zigler, Matthew L. Niemiller, Charles D.R. Stephen, Breanne N. Ayala, Marc A. Milne, Nicholas S. Gladstone, Annette S. Engel, John B. Jensen, Carlos D. Camp, James C. Ozier, and Alan Cressler. Biodiversity from caves and other subterranean habitats of Georgia, USA. Journal of Cave and Karst Studies, v. 82, no. 2, p. 125-167. DOI:10.4311/2019LSC0125 BIODIVERSITY FROM CAVES AND OTHER SUBTERRANEAN HABITATS OF GEORGIA, USA Kirk S. Zigler1C, Matthew L. Niemiller2, Charles D.R. Stephen3, Breanne N. Ayala1, Marc A. Milne4, Nicholas S. Gladstone5, Annette S. Engel6, John B. Jensen7, Carlos D. Camp8, James C. Ozier9, and Alan Cressler10 Abstract We provide an annotated checklist of species recorded from caves and other subterranean habitats in the state of Georgia, USA. We report 281 species (228 invertebrates and 53 vertebrates), including 51 troglobionts (cave-obligate species), from more than 150 sites (caves, springs, and wells). Endemism is high; of the troglobionts, 17 (33 % of those known from the state) are endemic to Georgia and seven (14 %) are known from a single cave. We identified three biogeographic clusters of troglobionts. Two clusters are located in the northwestern part of the state, west of Lookout Mountain in Lookout Valley and east of Lookout Mountain in the Valley and Ridge. In addition, there is a group of tro- globionts found only in the southwestern corner of the state and associated with the Upper Floridan Aquifer. At least two dozen potentially undescribed species have been collected from caves; clarifying the taxonomic status of these organisms would improve our understanding of cave biodiversity in the state. -

The Millipedes and Centipedes of Chiapas Amber
14 4 ANNOTATED LIST OF SPECIES Check List 14 (4): 637–646 https://doi.org/10.15560/14.4.637 The millipedes and centipedes of Chiapas amber Francisco Riquelme1, Miguel Hernández-Patricio2 1 Laboratorio de Sistemática Molecular. Escuela de Estudios Superiores del Jicarero, Universidad Autónoma del Estado de Morelos, Jicarero C.P. 62909, Morelos, Mexico. 2 Subcoordinación de Inventarios Bióticos, Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Tlalpan C.P. 14010, Mexico City, Mexico. Corresponding author: Francisco Riquelme, [email protected] Abstract An inventory of fossil millipedes (class Diplopoda) and centipedes (class Chilopoda) from Miocene Chiapas amber, Mexico, is presented, with the inclusion of new records. For Diplopoda, 34 members are enumerated, for which 31 are described as new fossil records of the orders Siphonophorida Newport, 1844, Spirobolida Bollman, 1893, Polydesmida Leach, 1895, Stemmiulida Pocock, 1894, and the superorder Juliformia Attems, 1926. For Chilopoda 8 fossils are listed, for which 3 are new records of the order Geophilomorpha Pocock, 1895 and 2 are of the order Scolopendromorpha Pocock, 1895. Key words Miocene, Mexico, Diplopoda, Chilopoda. Academic editor: Peter Dekker | Received 14 May 2018 | Accepted 26 July 2018 | Published 10 August 2018 Citation: Riquelme F, Hernández-Patricio F (2018) The millipedes and centipedes of Chiapas amber. Check List 14 (4): 637–646. https://doi. org/10.15560/14.4.637 Introduction Diplopoda fossil record worldwide (Edgecombe 2015). Two other centipedes have been reported in Ross et al. The extant species of millipedes and centipedes distributed (2016). Other records of millipedes have been mentioned across Mexico have been studied since the initial reports in the literature but are questionable because of a lack of Brand (1839) and Persbosc (1839).