United States Patent Office Patented June 28, 1966
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

Finding Refrigerant Leaks with the Chempro100i
Application Note: 108 Finding Refrigerant Leaks with the ChemPro100i The ChemPro100i is a great sniffer for halocarbon refrigerants, often generically referred to as “Freon” because of its multiple sensors and broad sniffing capability. As refrigerant sniffers are only carried by a small subset of first responders, the ChemPro100i can fill the role for those that don’t have a refrigerant sniffer. As a bonus, if someone suspects that it may be a refrigerant leak, and it turns out to be something else, the wide range of detectable gases and vapors seen by the ChemPro100i means that it will most likely find the unexpected gas/vapor too. The ChemPro100i’s Sensors The ChemPro100i uses a suite of seven sensors including an aspirated Ion Mobility Spectroscopy (IMS), five metal oxide sensors and a field effect sensor to detect, characterize, and even identify, some gases and vapors. Using this suite of sensors, the ChemPro100i can find halocarbon refrigerant leaks using its “Trend” or “sniffer” screen. As one gets closer to the refrigerant leak, the trend line will Using other Sniffers for increase. This is a non-quantifiable reading Refrigerants that does not directly correlate with parts per Most dedicated refrigerant detectors use a million (ppm), but the fast response of the Metal Oxide Sensor (MOS) that is doped to ChemPro100i to refrigerants provides one with be relatively specific to the halogenated the means of quickly finding a refrigerant leak. hydrocarbons (halogens) that are the hallmark of most refrigerants. MOS sensors Increase in the Trend line leads you to the leak are non-linear and not suitable for quantification of refrigerants, but they provide sensitive and fast response to halocarbon refrigerants. -

Halocarbon 32 (Difluoromethane) CH 2F2
Halocarbon 32 (Difluoromethane) CH 2F2 Grade ULSI 4N ULTIMA 4N8 Purity, % 99.99 99.998 Nitrogen ≤40 ppmv ≤5 ppmv Oxygen ≤10 ppmv ≤2 ppmv Carbon Dioxide ≤15 ppmv ≤1 ppmv Methane ≤2 ppmv ≤1 ppmv Water ≤5 ppmv ≤1 ppmv Carbon Tetrafluoride ≤5 ppmv ≤1 ppmv Other Organics ≤100 ppmv ≤10 ppmv Carbon Monoxide ≤1 ppmv Acidity as HF ≤0.1 ppmw • A lot analysis is provided for each order – Individual analysis is also available upon request. • Other Organics = H 12 , H 22 , H 23 , H 134a Internal Volume Liters 43.8 17.1 7.3 R E Cylinder Sizes >> QF GF UF D N I L lbs 64 25 11 Y Content C kg 29.1 11.35 5 Change Point* lbs 4.3 1.7 0.7 *Recommended Cylinder Change Point at NTP, based on Phase Break, or the amount of product left in the cylinder when the liquid phase has completely evaporated and only gaseous product is left (estimate based on ideal gas behavior). DOT Shipping Name Difluoromethane Shipped as P I DOT Classification 2.1 (Flammable Gas) H S Liquefied DOT Label FLAMMABLE GAS Gas UN Number UN 3252 Cylinder Pressure 207 psig Temp, °C 0.0 15.5 21.0 32.2 43.3 A Vapor T A @NTP 15.6 atm Press, psig 102 172 207 279 372 D 3 Pressure L Specific Volume 0.45 m /kg Temp, °F 32 60 70 90 110 A 3 C NTP = 21°C or 70°F and 101.3 kPa or 1 atm I @NTP 7.2 ft /lb N H CAS No 75-10-5 C E T CGA/DISS/JIS 350/724/W22-14L Molecular Weight 52 g/mol Nominal Diameter (OD)xHeight* Material of Construction Cylinder Treatment cm Inches Cylinder Valve ® QF ULTRA-LINE 23x130/134/143 9x51/52.5/56 CS SS ® GF ULTRA-LINE 23x66/70/79 9x26/27.5/31 CS SS ® UF ULTRA-LINE 15x51/55/64 6x19/20.5/24 CS SS *Height is reported as the distance from the bottom of the cylinder to the cylinder neck/ center of the valve outlet/ top of the handwheel CS: Carbon Steel SS: Stainless Steel WARNING: This product can expose you to chemicals including Carbon Monoxide, which is known to the State of California to cause birth defects or other reproductive harm. -

"Fluorine Compounds, Organic," In: Ullmann's Encyclopedia Of
Article No : a11_349 Fluorine Compounds, Organic GU¨ NTER SIEGEMUND, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany WERNER SCHWERTFEGER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany ANDREW FEIRING, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States BRUCE SMART, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States FRED BEHR, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States HERWARD VOGEL, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States BLAINE MCKUSICK, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States 1. Introduction....................... 444 8. Fluorinated Carboxylic Acids and 2. Production Processes ................ 445 Fluorinated Alkanesulfonic Acids ...... 470 2.1. Substitution of Hydrogen............. 445 8.1. Fluorinated Carboxylic Acids ......... 470 2.2. Halogen – Fluorine Exchange ......... 446 8.1.1. Fluorinated Acetic Acids .............. 470 2.3. Synthesis from Fluorinated Synthons ... 447 8.1.2. Long-Chain Perfluorocarboxylic Acids .... 470 2.4. Addition of Hydrogen Fluoride to 8.1.3. Fluorinated Dicarboxylic Acids ......... 472 Unsaturated Bonds ................. 447 8.1.4. Tetrafluoroethylene – Perfluorovinyl Ether 2.5. Miscellaneous Methods .............. 447 Copolymers with Carboxylic Acid Groups . 472 2.6. Purification and Analysis ............. 447 8.2. Fluorinated Alkanesulfonic Acids ...... 472 3. Fluorinated Alkanes................. 448 8.2.1. Perfluoroalkanesulfonic Acids -

Safety Data Sheet Tetrafluoromethane
Safety Data Sheet Tetrafluoromethane Section 1: Product and Company Identification Middlesex Gases & Technologies 292 Second Street P.O. Box 490249 Everett, MA 02149 (617) 387-5050 (800) 649-6704 Fax (617) 387-3537 http://www.middlesexgases.com/ Product Code: Tetrafluoromethane Section 2: Hazards Identification Warning Hazard Classification: Gases Under Pressure Hazard Statements: Contains gas under pressure; may explode if heated Precautionary Statements Storage: Protect from sunlight. Store in well-ventilated place. Section 3: Composition/Information on Ingredients CAS # 75-73-0 Middlesex Gases & Technologies page 1 of 5 Generated by the SDS Manager from AsteRisk, LLC. All Rights Reserved Generated: 06/01/2015 Chemical Substance Chemical Trade Names Family TETRAFLUOROMETHANE halogenated, CARBON TETRAFLUORIDE; CARBON FLUORIDE (CF4); CARBON FLUORIDE; FC 14; aliphatic PERFLUOROMETHANE; R 14; R 14 (REFRIGERANT); METHANE, TETRAFLUORO-; FREON 14; TETRAFLUOROCARBON; UN 1982; CF4 Section 4: First Aid Measures Skin Contact Eye Contact Ingestion Inhalation Note to Physicians If frostbite or freezing occur, Wash eyes immediately with If a large If adverse effects occur, remove to For immediately flush with plenty of large amounts of water, amount is uncontaminated area. Give artificial inhalation, lukewarm water (105-115 F; 41-46 occasionally lifting upper and swallowed, get respiration if not breathing. If consider C). DO NOT USE HOT WATER. If lower lids, until no evidence of medical breathing is difficult, oxygen should oxygen. warm water is not available, gently chemical remains. Get attention. be administered by qualified wrap affected parts in blankets. Get medical attention immediately. personnel. Get immediate medical immediate medical attention. attention. Section 5: Fire Fighting Measures Suitable Extinguishing Media Products of Combustion Protection of Firefighters Non-flammable. -

Refrigerants
Related Commercial Resources CHAPTER 29 REFRIGERANTS Refrigerant Properties .................................................................................................................. 29.1 Refrigerant Performance .............................................................................................................. 29.6 Safety ............................................................................................................................................. 29.6 Leak Detection .............................................................................................................................. 29.6 Effect on Construction Materials .................................................................................................. 29.9 EFRIGERANTS are the working fluids in refrigeration, air- cataracts, and impaired immune systems. It also can damage sensi- R conditioning, and heat-pumping systems. They absorb heat tive crops, reduce crop yields, and stress marine phytoplankton (and from one area, such as an air-conditioned space, and reject it into thus human food supplies from the oceans). In addition, exposure to another, such as outdoors, usually through evaporation and conden- UV radiation degrades plastics and wood. sation, respectively. These phase changes occur both in absorption Stratospheric ozone depletion has been linked to the presence of and mechanical vapor compression systems, but not in systems oper- chlorine and bromine in the stratosphere. Chemicals with long ating on a gas cycle using a -

Inventory of US Greenhouse Gas Emissions and Sinks: 1990-2015
ANNEX 6 Additional Information 6.1. Global Warming Potential Values Global Warming Potential (GWP) is intended as a quantified measure of the globally averaged relative radiative forcing impacts of a particular greenhouse gas. It is defined as the cumulative radiative forcing–both direct and indirect effectsintegrated over a specific period of time from the emission of a unit mass of gas relative to some reference gas (IPCC 2007). Carbon dioxide (CO2) was chosen as this reference gas. Direct effects occur when the gas itself is a greenhouse gas. Indirect radiative forcing occurs when chemical transformations involving the original gas produce a gas or gases that are greenhouse gases, or when a gas influences other radiatively important processes such as the atmospheric lifetimes of other gases. The relationship between kilotons (kt) of a gas and million metric tons of CO2 equivalents (MMT CO2 Eq.) can be expressed as follows: MMT MMT CO2 Eq. kt of gas GWP 1,000 kt where, MMT CO2 Eq. = Million metric tons of CO2 equivalent kt = kilotons (equivalent to a thousand metric tons) GWP = Global warming potential MMT = Million metric tons GWP values allow policy makers to compare the impacts of emissions and reductions of different gases. According to the IPCC, GWP values typically have an uncertainty of 35 percent, though some GWP values have larger uncertainty than others, especially those in which lifetimes have not yet been ascertained. In the following decision, the parties to the UNFCCC have agreed to use consistent GWP values from the IPCC Fourth Assessment Report (AR4), based upon a 100 year time horizon, although other time horizon values are available (see Table A-263). -

Airgas USA, LLC and Its Affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253
SAFETY DATA SHEET Halocarbon R-14 (Tetrafluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-14 (Tetrafluoromethane) Chemical name : carbon tetrafluoride Other means of : arcton 0; carbon fluoride; f 14; fc 14; freon 14; halon 14; methane, tetrafluoro-; identification perfluoromethane; r 14; r 14 (refrigerant); tetrafluoromethane; carbon tetrafluoride Product use : Synthetic/Analytical chemistry. Synonym : arcton 0; carbon fluoride; f 14; fc 14; freon 14; halon 14; methane, tetrafluoro-; perfluoromethane; r 14; r 14 (refrigerant); tetrafluoromethane; carbon tetrafluoride SDS # : 001051 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 Emergency telephone : 1-866-734-3438 number (with hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Use and store only outdoors or in a well ventilated place. -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane -

Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge Brigham Young University
Brigham Young University BYU ScholarsArchive All Theses and Dissertations 2017-12-01 Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge Brigham Young University Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Chemical Engineering Commons BYU ScholarsArchive Citation Hogge, Joseph Wallace, "Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities" (2017). All Theses and Dissertations. 6634. https://scholarsarchive.byu.edu/etd/6634 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in All Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacities Joseph Wallace Hogge A dissertation submitted to the faculty of Brigham Young University In partial fulfillment of the requirements for the degree of Doctor of Philosophy W. Vincent Wilding, Chair Thomas A. Knotts Dean Wheeler Thomas H. Fletcher John D. Hedengren Department of Chemical Engineering Brigham Young University Copyright © 2017 Joseph Wallace Hogge All Rights Reserved ABSTRACT Improving Thermodynamic Consistency Among Vapor Pressure, Heat of Vaporization, and Liquid and Ideal Gas Heat Capacity Joseph Wallace Hogge Department of Chemical Engineering, BYU Doctor of Philosophy Vapor pressure ( ), heat of vaporization ( ), liquid heat capacity ( ), and ideal gas heat capacity ( ) are important properties for process design and optimization. This work vap Δvap focuses on improving the thermodynamic consistency and accuracy of the aforementioned properties since these can drastically affect the reliability, safety, and profitability of chemical processes. -

ASPEN Refrigerants, Inc
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 000503 Supplier's details : ASPEN Refrigerants, Inc. 38-18 33rd Street Long Island City, NY 11101 1800-473-3766 Emergency telephone 1-800-424-9300 number (with 24 hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back-flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Use and store only outdoors or in a well-ventilated place. Response : Not applicable. Storage : Protect from sunlight. Protect from sunlight when ambient temperature exceeds 52°C/125°F. Store in a well-ventilated place. Disposal : Refer to manufacturer/supplier for information on recovery/recycling. -
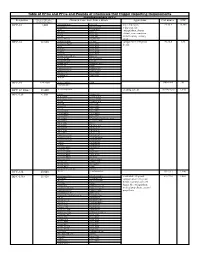
Table of Hfcs and Pfcs and Pounds of Chemicals That Trigger
Table of HFCs and PFCs and Pounds of Chemicals that Trigger Reporting Requirements Hydrofluorocarbons (HFCs) Designation Pounds of Substance = Chemical Name, Trade Names, Blends Applications CAS number GWP 10,000 metric tons Ce HFC-23 1,480 trifluoromethane Suva 95 low temperature 75-46-7 14,800 fluoroform Genetron 503 refrigerant, fire R-23 Genetron 23 extinguishant, plasma Freon 23 Klea 23 FE-13 Klea 508 etchant, semi-conductor FE-36 Klea 5R3 manufacturing cleaning Forane FX 220 NARM 503 agent HFC-32 32,660 difluoromethane R-407C component in refrigerant 75-10-5 675 methylene fluoride Klea 407A blends R-32 Klea 407B R-410A Klea 407C R-407C Klea 407D Freon 32 Klea 410A Forane 410A (AZ-20) Klea 32 Forane FX 40 Genetron 407C Forane FX 220 AZ-20 Forane 407C EcoloAce 407c Forane 32 HX4 Asahiklin SA-39 Solkane 407C Asahiklin SA-45 Solkane 410 Meforex 98 Suva 9000 Meforex 32 Suva 9100 R-410A HFC-41 227,280 methyl fluoride R-41 593-53-3 97 fluoromethane HFC-43-10mee 13,440 decafluoropentane cleaning solvent 138495-42-8 1,640 HFC-125 6,300 ethane Forane 507 pentafluoro Forane FX10 pentafluoroethane Forane FX40 R-125 Forane FX70 FC-125 Suva 125 Freon 125 Suva 9000 FE-25 Suva 9100 Arcton 402A Suva HP62 Arcton 402B Suva HP80 Arcton 408A Suva HP81 Klea 404A Cooltop R-134a Replacement Klea 407A EcoloAce 404a Klea 407B Di 44 Klea 407C Meforex 125 Klea 407D Meforex 55 Klea 410A Meforex 57 Klea 507A Meforex 98 Asahiklin SA-28 ISCEON 29 Asahiklin SA-39 ISCEON 404A Asahiklin SA-45 ISCEON 507 AZ-20 ISCEON 59 AZ-50 ISCEON 79 Genetron 404A ISCEON 89 Genetron