Inhibition of Methane Oxidation by Methylococcus Capsulatus with Hydrochlorofluorocarbons and Fluorinated Methanes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Finding Refrigerant Leaks with the Chempro100i
Application Note: 108 Finding Refrigerant Leaks with the ChemPro100i The ChemPro100i is a great sniffer for halocarbon refrigerants, often generically referred to as “Freon” because of its multiple sensors and broad sniffing capability. As refrigerant sniffers are only carried by a small subset of first responders, the ChemPro100i can fill the role for those that don’t have a refrigerant sniffer. As a bonus, if someone suspects that it may be a refrigerant leak, and it turns out to be something else, the wide range of detectable gases and vapors seen by the ChemPro100i means that it will most likely find the unexpected gas/vapor too. The ChemPro100i’s Sensors The ChemPro100i uses a suite of seven sensors including an aspirated Ion Mobility Spectroscopy (IMS), five metal oxide sensors and a field effect sensor to detect, characterize, and even identify, some gases and vapors. Using this suite of sensors, the ChemPro100i can find halocarbon refrigerant leaks using its “Trend” or “sniffer” screen. As one gets closer to the refrigerant leak, the trend line will Using other Sniffers for increase. This is a non-quantifiable reading Refrigerants that does not directly correlate with parts per Most dedicated refrigerant detectors use a million (ppm), but the fast response of the Metal Oxide Sensor (MOS) that is doped to ChemPro100i to refrigerants provides one with be relatively specific to the halogenated the means of quickly finding a refrigerant leak. hydrocarbons (halogens) that are the hallmark of most refrigerants. MOS sensors Increase in the Trend line leads you to the leak are non-linear and not suitable for quantification of refrigerants, but they provide sensitive and fast response to halocarbon refrigerants. -

Halocarbon 32 (Difluoromethane) CH 2F2
Halocarbon 32 (Difluoromethane) CH 2F2 Grade ULSI 4N ULTIMA 4N8 Purity, % 99.99 99.998 Nitrogen ≤40 ppmv ≤5 ppmv Oxygen ≤10 ppmv ≤2 ppmv Carbon Dioxide ≤15 ppmv ≤1 ppmv Methane ≤2 ppmv ≤1 ppmv Water ≤5 ppmv ≤1 ppmv Carbon Tetrafluoride ≤5 ppmv ≤1 ppmv Other Organics ≤100 ppmv ≤10 ppmv Carbon Monoxide ≤1 ppmv Acidity as HF ≤0.1 ppmw • A lot analysis is provided for each order – Individual analysis is also available upon request. • Other Organics = H 12 , H 22 , H 23 , H 134a Internal Volume Liters 43.8 17.1 7.3 R E Cylinder Sizes >> QF GF UF D N I L lbs 64 25 11 Y Content C kg 29.1 11.35 5 Change Point* lbs 4.3 1.7 0.7 *Recommended Cylinder Change Point at NTP, based on Phase Break, or the amount of product left in the cylinder when the liquid phase has completely evaporated and only gaseous product is left (estimate based on ideal gas behavior). DOT Shipping Name Difluoromethane Shipped as P I DOT Classification 2.1 (Flammable Gas) H S Liquefied DOT Label FLAMMABLE GAS Gas UN Number UN 3252 Cylinder Pressure 207 psig Temp, °C 0.0 15.5 21.0 32.2 43.3 A Vapor T A @NTP 15.6 atm Press, psig 102 172 207 279 372 D 3 Pressure L Specific Volume 0.45 m /kg Temp, °F 32 60 70 90 110 A 3 C NTP = 21°C or 70°F and 101.3 kPa or 1 atm I @NTP 7.2 ft /lb N H CAS No 75-10-5 C E T CGA/DISS/JIS 350/724/W22-14L Molecular Weight 52 g/mol Nominal Diameter (OD)xHeight* Material of Construction Cylinder Treatment cm Inches Cylinder Valve ® QF ULTRA-LINE 23x130/134/143 9x51/52.5/56 CS SS ® GF ULTRA-LINE 23x66/70/79 9x26/27.5/31 CS SS ® UF ULTRA-LINE 15x51/55/64 6x19/20.5/24 CS SS *Height is reported as the distance from the bottom of the cylinder to the cylinder neck/ center of the valve outlet/ top of the handwheel CS: Carbon Steel SS: Stainless Steel WARNING: This product can expose you to chemicals including Carbon Monoxide, which is known to the State of California to cause birth defects or other reproductive harm. -

Safety Data Sheet Tetrafluoromethane
Safety Data Sheet Tetrafluoromethane Section 1: Product and Company Identification Middlesex Gases & Technologies 292 Second Street P.O. Box 490249 Everett, MA 02149 (617) 387-5050 (800) 649-6704 Fax (617) 387-3537 http://www.middlesexgases.com/ Product Code: Tetrafluoromethane Section 2: Hazards Identification Warning Hazard Classification: Gases Under Pressure Hazard Statements: Contains gas under pressure; may explode if heated Precautionary Statements Storage: Protect from sunlight. Store in well-ventilated place. Section 3: Composition/Information on Ingredients CAS # 75-73-0 Middlesex Gases & Technologies page 1 of 5 Generated by the SDS Manager from AsteRisk, LLC. All Rights Reserved Generated: 06/01/2015 Chemical Substance Chemical Trade Names Family TETRAFLUOROMETHANE halogenated, CARBON TETRAFLUORIDE; CARBON FLUORIDE (CF4); CARBON FLUORIDE; FC 14; aliphatic PERFLUOROMETHANE; R 14; R 14 (REFRIGERANT); METHANE, TETRAFLUORO-; FREON 14; TETRAFLUOROCARBON; UN 1982; CF4 Section 4: First Aid Measures Skin Contact Eye Contact Ingestion Inhalation Note to Physicians If frostbite or freezing occur, Wash eyes immediately with If a large If adverse effects occur, remove to For immediately flush with plenty of large amounts of water, amount is uncontaminated area. Give artificial inhalation, lukewarm water (105-115 F; 41-46 occasionally lifting upper and swallowed, get respiration if not breathing. If consider C). DO NOT USE HOT WATER. If lower lids, until no evidence of medical breathing is difficult, oxygen should oxygen. warm water is not available, gently chemical remains. Get attention. be administered by qualified wrap affected parts in blankets. Get medical attention immediately. personnel. Get immediate medical immediate medical attention. attention. Section 5: Fire Fighting Measures Suitable Extinguishing Media Products of Combustion Protection of Firefighters Non-flammable. -

Airgas USA, LLC and Its Affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253
SAFETY DATA SHEET Halocarbon R-14 (Tetrafluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-14 (Tetrafluoromethane) Chemical name : carbon tetrafluoride Other means of : arcton 0; carbon fluoride; f 14; fc 14; freon 14; halon 14; methane, tetrafluoro-; identification perfluoromethane; r 14; r 14 (refrigerant); tetrafluoromethane; carbon tetrafluoride Product use : Synthetic/Analytical chemistry. Synonym : arcton 0; carbon fluoride; f 14; fc 14; freon 14; halon 14; methane, tetrafluoro-; perfluoromethane; r 14; r 14 (refrigerant); tetrafluoromethane; carbon tetrafluoride SDS # : 001051 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 Emergency telephone : 1-866-734-3438 number (with hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Use and store only outdoors or in a well ventilated place. -

SROC Annex V
Annex V Major Chemical Formulae and Nomenclature This annex presents the formulae and nomenclature for halogen-containing species and other species that are referred to in this report (Annex V.1). The nomenclature for refrigerants and refrigerant blends is given in Annex V.2. V.1 Substances by Groupings V.1.1 Halogen-Containing Species V.1.1.1 Inorganic Halogen-Containing Species Atomic chlorine Cl Atomic bromine Br Molecular chlorine Cl2 Molecular bromine Br2 Chlorine monoxide ClO Bromine monoxide BrO Chlorine radicals ClOx Bromine radicals BrOx Chloroperoxy radical ClOO Bromine nitrate BrONO2, BrNO3 Dichlorine peroxide (ClO dimer) (ClO)2, Cl2O2 Potassium bromide KBr Hydrogen chloride (Hydrochloric acid) HCl Inorganic chlorine Cly Antimony pentachloride SbCl5 Atomic fluorine F Molecular fluorine F2 Atomic iodine I Hydrogen fluoride (Hydrofluoric acid) HF Molecular iodine I2 Sulphur hexafluoride SF6 Nitrogen trifluoride NF3 IPCC Boek (dik).indb 467 15-08-2005 10:57:13 468 IPCC/TEAP Special Report: Safeguarding the Ozone Layer and the Global Climate System V.1.1.2 Halocarbons For each halocarbon the following information is given in columns: • Chemical compound [Number of isomers]1 (or common name) • Chemical formula • CAS number2 • Chemical name (or alternative name) V.1.1.2.1 Chlorofluorocarbons (CFCs) CFC-11 CCl3F 75-69-4 Trichlorofluoromethane CFC-12 CCl2F2 75-71-8 Dichlorodifluoromethane CFC-13 CClF3 75-72-9 Chlorotrifluoromethane CFC-113 [2] C2Cl3F3 Trichlorotrifluoroethane CCl FCClF 76-13-1 CFC-113 2 2 1,1,2-Trichloro-1,2,2-trifluoroethane -
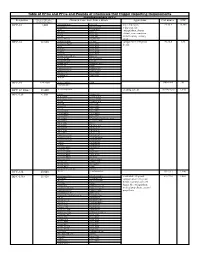
Table of Hfcs and Pfcs and Pounds of Chemicals That Trigger
Table of HFCs and PFCs and Pounds of Chemicals that Trigger Reporting Requirements Hydrofluorocarbons (HFCs) Designation Pounds of Substance = Chemical Name, Trade Names, Blends Applications CAS number GWP 10,000 metric tons Ce HFC-23 1,480 trifluoromethane Suva 95 low temperature 75-46-7 14,800 fluoroform Genetron 503 refrigerant, fire R-23 Genetron 23 extinguishant, plasma Freon 23 Klea 23 FE-13 Klea 508 etchant, semi-conductor FE-36 Klea 5R3 manufacturing cleaning Forane FX 220 NARM 503 agent HFC-32 32,660 difluoromethane R-407C component in refrigerant 75-10-5 675 methylene fluoride Klea 407A blends R-32 Klea 407B R-410A Klea 407C R-407C Klea 407D Freon 32 Klea 410A Forane 410A (AZ-20) Klea 32 Forane FX 40 Genetron 407C Forane FX 220 AZ-20 Forane 407C EcoloAce 407c Forane 32 HX4 Asahiklin SA-39 Solkane 407C Asahiklin SA-45 Solkane 410 Meforex 98 Suva 9000 Meforex 32 Suva 9100 R-410A HFC-41 227,280 methyl fluoride R-41 593-53-3 97 fluoromethane HFC-43-10mee 13,440 decafluoropentane cleaning solvent 138495-42-8 1,640 HFC-125 6,300 ethane Forane 507 pentafluoro Forane FX10 pentafluoroethane Forane FX40 R-125 Forane FX70 FC-125 Suva 125 Freon 125 Suva 9000 FE-25 Suva 9100 Arcton 402A Suva HP62 Arcton 402B Suva HP80 Arcton 408A Suva HP81 Klea 404A Cooltop R-134a Replacement Klea 407A EcoloAce 404a Klea 407B Di 44 Klea 407C Meforex 125 Klea 407D Meforex 55 Klea 410A Meforex 57 Klea 507A Meforex 98 Asahiklin SA-28 ISCEON 29 Asahiklin SA-39 ISCEON 404A Asahiklin SA-45 ISCEON 507 AZ-20 ISCEON 59 AZ-50 ISCEON 79 Genetron 404A ISCEON 89 Genetron -

Tetrafluoromethane, Refrigerant Gas R14
Tetrafluoromethane, Refrigerant Gas R14 Safety Data Sheet P-4665 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Issue date: 01/01/1980 Revision date: 02/09/2021 Supersedes: 10/24/2016 Version: 1.0 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Trade name : carbon tetrafluoride Chemical name : Tetrafluoromethane CAS-No. : 75-73-0 Formula : CF4 Other means of identification : Tetrafluoromethane (R14), Carbon tetrafluoride, F-14, refrigerant gas R14, perfluoromethane, halon-14 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Industrial use; Use as directed. 1.3. Details of the supplier of the safety data sheet Praxair, Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA T 1-800-772-9247 (1-800-PRAXAIR) - F 1-716-879-2146 www.praxair.com 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS US classification Simple asphyxiant SIAS Press. Gas (Liq.) H280 2.2. Label elements GHS US labeling Hazard pictograms (GHS US) : GHS04 Signal word (GHS US) : Warning Hazard statements (GHS US) : H280 - CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED OSHA-H01 - MAY DISPLACE OXYGEN AND CAUSE RAPID SUFFOCATION. CGA-HG01 - MAY CAUSE FROSTBITE. Precautionary statements (GHS US) : P202 - Do not handle until all safety precautions have been read and understood. -

SAFETY DATA SHEET Halocarbon R-14 (Tetrafluoromethane)
SAFETY DATA SHEET Halocarbon R-14 (Tetrafluoromethane) Section 1. Identification GHS product identifier : Halocarbon R-14 (Tetrafluoromethane) Chemical name : carbon tetrafluoride Other means of : arcton 0; carbon fluoride; f 14; fc 14; freon 14; halon 14; methane, tetrafluoro-; identification perfluoromethane; r 14; r 14 (refrigerant); tetrafluoromethane; carbon tetrafluoride Product use : Synthetic/Analytical chemistry. Synonym : arcton 0; carbon fluoride; f 14; fc 14; freon 14; halon 14; methane, tetrafluoro-; perfluoromethane; r 14; r 14 (refrigerant); tetrafluoromethane; carbon tetrafluoride SDS # : 000014 Supplier's details : ASPEN Refrigerants, Inc. 38-18 33rd Street Long Island City, NY 11101 1-800-473-3766 Emergency telephone :1-800-424-9300 number (with 24 hours of operation) Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back-flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Use and store only outdoors or in a well-ventilated place. Response : Not applicable. -

Safety Data Sheet Tetrafluoromethane 1. Identification of the Substance/Compound and Company 2. Hazards Identification
Revised edition no :5 SAFETY DATA SHEET ACCORDING TO REGULATION 1907/2006 Date : 08.10.2019 TETRAFLUOROMETHANE 1. IDENTIFICATION OF THE SUBSTANCE/COMPOUND AND COMPANY 1.1. Product identifier Name: Tetrafluoromethane (Refrigerant-14) Chemical name Tetrafluoromethane Synonyms: Refrigerant-14, carbon tetrafluoride, Freon-14, R-14, metforane, perfluoromethane, refrigerant gas R-14 Chemical formula: CF4 Molecular weight: 88,005 EC number 200-896-5 (EINECS) REACH Pre-Registration № Reference number 05-2114096911-42-0000 C&L bulk notification Reference number 02-2119708818-29-0000 CAS number 75-73-0 1.2 Relevant identified uses of Applied as refrigerant for the production of ultralow temperatures the substance or mixture, and -120 ÷ -150 0C (-180 ÷ -240 0F), as stabilizer of nitrogen decomposition and uses advised against flame inhibitor /3/. 1.2.1 Identified uses Applied as reagent for dry etching by producing of integrated circuit, as thinner by carrying out chemical reactions, as reagent for the production of fluorine organic products/3/. 1.2.2 Uses advised against unknown 1.3 Details of the supplier of the safety data sheet Manufacturer Joint Stock Company «HaloPolymer Perm» 614042, Russia, Perm, ul. Lasvinskaya 98 Phone № +7(342) 250-61-50 www.halopolymer.ru Only REACH representative in JSC «HaloPolymer Perm» (Submitting legal entity URALCHEM Assist EU: GmbH) Johannssenstrasse 10 30159, Hannover, Germany Tel: +49 511 45 99 444 1.4 Emergency telephone: +7-342-282-85-45 (24 hours) Great Britain +44 (0) 203 394 9870 (24/7) USA 1-877 271 7077 2. HAZARDS IDENTIFICATION 2.1 Classification of the substance 2.1.1 Regulation (EC) Liquefied gas, H280 No 1272/2008 [CLP/GHS] 2.1.2. -

“Fluorocarbons”
“Fluorocarbons” -Alex J. Roche A Graduate Level Advanced Organic Course Rutgers, The State University of New Jersey 56:160:519 Office: Sci 311 Labs: Sci 309, 328F, 304A [email protected] Tel: (856) 225-6166 Fax: (856) 225-6506 http://crab.rutgers.edu/~alroche/ “Fluorine leaves nobody indifferent; it inflames emotions be that affections or aversions. As a substituent, it is rarely boring, always good for a surprise, but often completely unpredictable” – Manfred Schlosser, 1998. 1 Fluorocarbons Fluorocarbons are organic compounds that contain fluorine, and they are (almost always) synthetic or man made compounds. Compounds bearing a C-F bond do not occur in nature (almost true). Thus the replacement of a C-H bond with a C-F bond gives rise to a potentially vast man made organic chemistry. Adds an entirely synthetic extra dimension to Organic Chemistry. Fluorocarbons can Provide: New Chemistry Unique Chemistry (Special Effects) New materials New Applications 2 Organofluorine Chemists (should) Aim to: -Develop the Organic Chemistry of compounds that contain Fluorine -Encourage application by collaboration with industry -Integrate the subject on a mechanistic basis with modern organic chemistry (get to a level of understanding so F is predictably unique) 3 History Hydrogen Fluoride was first reported by Scheele in 1771. The first reported synthesis of an organofluorine compound was in 1836, when Dumas and Peligot reported the synthesis of fluoromethane. It was in 1886 that Henri Moissan prepared and isolated molecular elemental fluorine gas (F2). Moissan in 1890 erroneously reported the isolation of carbon tetrafluoride as a product of the reaction of fluorine and carbon. -

Sustainable Degradation of Carbon Tetrafluoride to Non-Corrosive Useful Products by Incorporating Reduced Electron Mediator Within Electro- Scrubbing
Accepted Manuscript Title: Sustainable degradation of carbon tetrafluoride to non-corrosive useful products by incorporating reduced electron mediator within electro-scrubbing Authors: G. Muthuraman, A.G. Ramu, Y.H. Cho, E.J. McAdam, I.S. Moon PII: S1226-086X(18)30095-9 DOI: https://doi.org/10.1016/j.jiec.2018.02.025 Reference: JIEC 3885 To appear in: Received date: 27-12-2017 Revised date: 5-2-2018 Accepted date: 10-2-2018 Please cite this article as: G.Muthuraman, A.G.Ramu, Y.H.Cho, E.J.McAdam, I.S.Moon, Sustainable degradation of carbon tetrafluoride to non-corrosive useful products by incorporating reduced electron mediator within electro-scrubbing, Journal of Industrial and Engineering Chemistry https://doi.org/10.1016/j.jiec.2018.02.025 This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain. Sustainable degradation of carbon tetrafluoride to non-corrosive useful products by incorporating reduced electron mediator within electro- scrubbing a a b c a G. Muthuraman , A.G. Ramu , Y. H. Cho , E.J. McAdam , and I. S. Moon * aDepartment of Chemical Engineering, Sunchon National University, #255 Jungangno, Suncheon 540-742, Jeollanam-do, Rep. of Korea. bKorea Atomic Energy Research Institute, Yuseong P.O Box 105, Daejeon, Rep. -

CHAPTER 4 Industrial and Utilitarian Aspects of Fluorine Chemistry 3M MN04855110
CHAPTER 4 Industrial and Utilitarian Aspects of Fluorine Chemistry BY H. G. BRYCE f~Iinnesota ~Vlining and Manufacturing Company, St. Paul, Minnesota I. Introduction ....................................................... 297 II. Historical and Economic Factors ..................................... 298 III. Characteristic Properties of Fluorocarbons .............................. 302 A. Bond Energies and Bond Distances ............................... 302 B. Boiling Points and Melting Points ................................ 303 C. Surface Energy ................................................ 304 D. Stability ...................................................... 309 E. Electrical Properties ............................................ 312 F. Polarity of Oxides or Nitrides and Effect of Structure ............... 312 G. Solubility ..................................................... 314 IV. Refrigerants and Propellants ......................................... 316 A. Properties ..................................................... 317 B. Refrigeration Characteristics ..................................... 320 C. Applications for Specific Compounds ............................. 323 V. Heat Transfer Media ................................................ 323 A. Heat Transfer Processes ........................................ 323 B. Miniaturization ................................................ 324 C. Application of FIuorocarbon Fluids to Heat Transfer Problems ....... 325 D. Properties of Fluorocarbon Fluids ...............................