Common Metabolites Alcohol Paper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCA One Care Options Formulary
Commonwealth Care Alliance One Care Plan (Medicare-Medicaid Plan) 2021 List of Covered Drugs (Formulary) 30 Winter Street • Boston, MA 02108 PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN For more recent information or other questions, contact Commonwealth Care Alliance Member Services at 1-866-610-2273 (TTY: call MassRelay at 711), 8 a.m. – 8 p.m., 7 days a week, or visit www.commonwealthonecare.org H0137_CF2021 Approved Formulary: ID 00021588 • Version 13 • Updated on 08/01/2021 One Care Plan | 2021 List of Covered Drugs (Formulary) Introduction This document is called the List of Covered Drugs (also known as the Drug List). It tells you which prescription drugs, over-the-counter drugs and items are covered by Commonwealth Care Alliance. The Drug List also tells you if there are any special rules or restrictions on any drugs covered by One Care. Key terms and their definitions appear in the last chapter of the Member Handbook. Table of Contents A. Disclaimers ........................................................................................................................ 4 B. Frequently Asked Questions (FAQ) .................................................................................. 5 What prescription drugs are on the List of Covered Drugs? (We call the List of Covered Drugs the “Drug List” for short.) ................................................................... 5 B2. Does the Drug List ever change? ............................................................................... 5 B3. What happens when there is a change to the Drug List? ........................................... 6 B4. Are there any restrictions or limits on drug coverage or any required actions to take to get certain drugs? .................................................................................................. 7 B5. How will you know if the drug you want has limitations or if there are required actions to take to get the drug? ................................................................................. -

Download Author Version (PDF)
Organic & Biomolecular Chemistry Accepted Manuscript This is an Accepted Manuscript, which has been through the Royal Society of Chemistry peer review process and has been accepted for publication. Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available. You can find more information about Accepted Manuscripts in the Information for Authors. Please note that technical editing may introduce minor changes to the text and/or graphics, which may alter content. The journal’s standard Terms & Conditions and the Ethical guidelines still apply. In no event shall the Royal Society of Chemistry be held responsible for any errors or omissions in this Accepted Manuscript or any consequences arising from the use of any information it contains. www.rsc.org/obc Page 1 of 26 Organic & Biomolecular Chemistry Comparison of alternative nucleophiles for Sortase A-mediated bioconjugation and application in neuronal cell labelling Samuel Baera, Julie Nigro a,b, Mariusz P. Madej a, Rebecca M. Nisbet a,b, Randy Suryadinata a, Gregory Coia a, Lisa P. T. Hong a, Timothy E. Adams a, Charlotte C. Williams *a†, Stewart D. Nuttall a,b†. Manuscript aCSIRO Materials Science and Engineering, 343 Royal Parade, Parkville, Victoria, 3052, AUSTRALIA. bPreventative Health Flagship, 343 Royal Parade, Parkville, Victoria, 3052, AUSTRALIA. *Correspondence to: Charlotte C. Williams ([email protected] ) at CSIRO Materials Accepted Science and Engineering, 343 Royal Parade, Parkville, Victoria, 3052, AUSTRALIA; Ph: +61 3 9662 7100). -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

List of Toxic Chemicals Within the Glycol Ethers Category
United States Office of Environmental Revised December 2000 Environmental Protection Information EPA 745-R-00-004 Agency Washington, DC 20460 TOXICS RELEASE INVENTORY List of Toxic Chemicals within the Glycol Ethers Category Section 313 of the Emergency Planning and Community Right-to-Know Act (EPCRA) requires certain facilities manufacturing, processing, or otherwise using listed toxic chemicals to report their environmental releases of such chemicals annually. Beginning with the 1991 reporting year, such facilities also must report pollution prevention and recycling data for such chemicals, pursuant to section 6607 of the Pollution Prevention Act, 42 U.S.C. 13106. When enacted, EPCRA section 313 established an initial list of toxic chemicals that was comprised of more than 300 chemicals and 20 chemical categories. EPCRA section 313(d) authorizes EPA to add chemicals to or delete chemicals from the list, and sets forth criteria for these actions. CONTENTS Section 1. Introduction ...................................................... 3 Section 2. CAS Number List of Some Chemicals within the Glycol Ethers Category ........ 6 Section 3. CAS Number List of Some Mixtures That Contain Glycol Ethers within the Category .............................................. 185 Section 4. CAS Number List of Some Oligomeric or Polymeric Chemicals That Might Contain Glycol Ether Components within the Category .......................... 187 FOREWORD This document is an updated version of the previous document, EPA 745-R-99-006, June 1999. This version has the following updates: • The titles to Table 1 on page 6, Table 2 on page 185, and Table 3 on 187 are modified; and • The CAS number of second listing in Table 3 (Poly(oxy-1,2-ethanediyl), .alpha.- (phenylsulfonyl)-.omega.-methoxy-) on page 187 is changed from 7664-41-7 to 67584-43-4. -
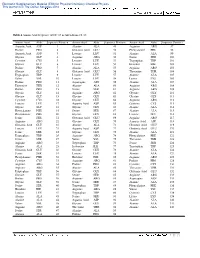
Table 2 Amino Acid Sequence of OC-17 As Taken from Ref. 28 Amino
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics This journal is © The Owner Societies 2012 Table 2 Amino Acid Sequence of OC-17 as taken from ref. 28 Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Amino Acid Abbr. Sequence Position Aspartic Acid ASP 1 Alanine ALA 49 Arginine ARG 97 Proline PRO 2 Glutamic Acid GLU 50 Phenyalanine PHE 98 Aspartic Acid ASP 3 Leucine LEU 51 Alanine ALA 99 Glycine GLY 4 Arginine ARG 52 Serine SER 100 Cysteine CYS 5 Leucine LEU 53 Tryptophan TRP 101 Glycine GLY 6 Leucine LEU 54 Histidine HIE 102 Proline PRO 7 Alanine ALA 55 Arginine ARG 103 Glycine GLY 8 Glutamic Acid GLU 56 Threonine THR 104 Tryptophan TRP 9 Leucine LEU 57 Alanine ALA 105 Valine VAL 10 Leucine LEU 58 Lysine LYS 106 Proline PRO 11 Asparagine ASN 59 Alanine ALA 107 Threonine THR 12 Alanine ALA 60 Arginine ARG 108 Proline PRO 13 Serine SER 61 Arginine ARG 109 Glycine GLY 14 Arginine ARG 62 Glycine GLY 110 Glycine GLY 15 Glycine GLY 63 Glycine GLY 111 Cysteine CYS 16 Glycine GLY 64 Arginine ARG 112 Leucine LEU 17 Aspartic Acid ASP 65 Cysteine CYS 113 Glycine GLY 18 Glycine GLY 66 Alanine ALA 114 Phenyalanine PHE 19 Serine SER 67 Alanine ALA 115 Phenyalanine PHE 20 Glycine GLY 68 Leucine LEU 116 Serine SER 21 Glutamic Acid GLU 69 Arginine ARG 117 Arginine ARG 22 Glycine GLY 70 Aspartic Acid ASP 118 Glutamic Acid GLU 23 Alanine ALA 71 Glutamic Acid GLU 119 Leucine LEU 24 Aspartic Acid ASP 72 Glutamic Acid GLU 120 Serine SER 25 Glycine GLY 73 Alanine ALA 121 Tryptophan TRP 26 Arginine ARG 74 Phenyalanine -

Utilization of Anthranilic and Nitrobenzoic Acids by Nocardia Opaca and a Flavobacteriurn
J. gen. Microbial. (1966),42, 219-235 Printed in Great Britain Utilization of Anthranilic and Nitrobenzoic Acids by Nocardia opaca and a Flavobacteriurn BY R. B. CAIN Depadnaent of Microbiology, OkEahoma State University, Stillwater, Oklahoma, U.S.A. and Department of Botany, University of Newcastle upon Tyne” (Received 11 May 1965, accepted 16 September 1965) SUMMARY Anthranilic and o-nitrobenzoic acids act as mutual inhibitors of both growth and substrate oxidation for Nocardia opaca and a flavobacterium which can utilize either substance as sole source of carbon, nitrogen and energy, Growth of the former bacterium on anthranilate induced, appar- ently simultaneously, both the transport system for anthranilate uptake and the enzymic mechanism necessary for its complete oxidation to CO, and NH3. Among the enzymes induced by anthranilate was the complete sequence that oxidizes catechol to /3-oxoadipate; this was absent from organisms grown in fumarate or glucose media. The properties of the first enzyme in this sequence, a catechol-l,2-oxygenase, differ in several features from those of the same enzyme induced in this bacterium by growth on o-nitrobenzoic acid. INTRODUCTION Anthranilic (o-aminobenzoic) acid is an important intermediary metabolite in both biosynthetic and catabolic pathways in micro-organisms. It serves for in- stance as a precursor for tryptophan in Aerobacter aerogews and Escherichia coli (Doy & Gibson, 1961), in Nmrospora CT~SSQ(Yanofsky, 1956; Yanofsky & Rach- meler, 1958) and in saccharomyces mutants (Lingens, Hildinger & Hellman, 1958). Hydroxylation of anthranilic acid, in the 3-position, has been observed with rat liver preparations (Wiss & Hellman, 1953) but no hydroxylation of anthranilic acid has been conclusively demonstrated in micro-organisms. -

Chemicals Required for the Illicit Manufacture of Drugs Table 1 SUBSTANCES in TABLES I and II of the 1988 CONVENTION
Chemicals Required 1. A variety of chemicals are used in the illicit manufacture of for the Illicit drugs. The United Nations Convention against Illicit Traffic in Manufacture of Drugs Narcotic Drugs and Psychotropic Substances of 1988 (1988 Convention) refers to “substances frequently used in the illicit manufacture of narcotic drugs and psychotropic substances”. Twenty-two such substances are listed in Tables I and II of the 1988 Convention as in force on 1st May, 1998. (See Table1) Table 1 Table I Table II SUBSTANCES IN N-Acetylanthranilic acid. Acetic anhydride TABLES I AND II OF Ephedrine Acetone THE 1988 Ergometrine Anthranilic acid CONVENTION Ergotamine Ethyl ether Isosafrole Hydrochloric acid* Lysergic acid Methyl ethyl ketone 3,4-methylenedioxyphenyl-2-propanone Phenylacetic acid 1-phenyl-2-propanone Piperidine Piperonal Potassium permanganate Pseudoephedrine Sulphuric acid* Safrole Toluene The salts of the substances in this Table The salts of the substances whenever the existence of such salts is in this Table whenever the possible. existence of such salts is possible. * The salts of hydrochloric acid and sulphuric acid are specifically excluded from Table II. U N D C P 11 The term “precursor” is used to indicate any of these substances in the two Tables. Chemicals used in the illicit manufacture of narcotic drugs and psychotropic substances are often described as precursors or essential chemicals, and these include true precursors, solvents, oxidising agents and other Chemicals used in the illicit manufacture of narcotic substances. Although the term is not drugs and psychotropic substances are often technically correct, it has become common described as precursors or essential chemicals, and practice to refer to all such substances as these include true precursors, solvents, oxidising “precursors”. -

The Fate of Arginine and Proline Carbon in Squid Tissuesl
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved The Fate of Arginine and Proline Carbon in Squid Tissuesl T. P. MOMMSEN,2 C. J. FRENCH,2 B. EMMETI,2 and P. W. HOCHACHKA2 ABSTRACT: The metabolism of proline and arginine was investigated in kidney, gill, and heart of the pelagic squid, Symplectoteuthis. The rates of CO2 release from 14C-proline exceeded the rates from 14C-arginine. The metabolic rate of arginine and proline was assessed by monitoring the incorporation of arginine-derived carbon into various intermediates. Arginine was metabolized, through ornithine, to proline as well as to glutamate and various subsequent derivatives (alanine, octopine, aspartate, and carboxylic acids). The same com ponents became labeled using 14C-proline as the starting substrate, but only the gill was capable ofconverting proline to arginine via the urea cycle. In addition, 14C-proline oxidation rates were high enough to exceed those of 14C-glucose in at least three tissues, kidney, heart, and inner mantle muscle. AT LEAST IN PART because ofthe large pool size data for heart, gill, and kidney from the squid, of free amino acids in cephalopod muscles Symplectoteuthis, showing the capacity for ar (e.g., see Hochachka, French, and Meredith ginine conversion to proline. The conversion 1978), interest recently has been focusing ofproline to arginine was measurable only in on their possible roles in energy metabolism. the gill. Although qualitatively similar to re During metabolic studies on the 1979 Alpha sults obtained with other species, these data Helix Cephalopod Expedition, relatively high also show some important, tissue-specific dif rates of CO2 release from arginine and ferences (Mommsen et al. -

PEREGRINO-THESIS-2017.Pdf (6.329Mb)
Biochemical studies in the elucidation of genes involved in tropane alkaloid production in Erythroxylum coca and Erythroxylum novogranatense by Olga P. Estrada, B. S. A Thesis In Chemical Biology Submitted to the Graduate Faculty of Texas Tech University in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCES Approved Dr. John C. D’Auria Chair of Committee Dr. David W. Nes Co-chair of Committee Mark Sheridan Dean of the Graduate School May, 2017 Copyright 2017, Olga P. Estrada Texas Tech University, Olga P. Estrada, May 2017 AKNOWLEDGMENTS I would like to thank my mentor and advisor Dr. John C. D’Auria, for providing me with the tools to become a scientist, and offering me his unconditional support. Thanks to the members of the D’Auria lab, especially Neill Kim and Benjamin Chavez for their aid during my experimental studies. And of course, thank you to my family for always giving me the strength to pursue my goals. ii Texas Tech University, Olga P. Estrada, May 2017 TABLE OF CONTENTS AKNOWLEDGMENTS ........................................................................................................... ii ABSTRACT ........................................................................................................................... v LIST OF TABLES ................................................................................................................. vi LIST OF FIGURES ............................................................................................................... vii CHAPTER I ......................................................................................................................... -

Arginine Is Synthesized from Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates
0031-3998/11/6901-0046 Vol. 69, No. 1, 2011 PEDIATRIC RESEARCH Printed in U.S.A. Copyright © 2010 International Pediatric Research Foundation, Inc. Arginine Is Synthesized From Proline, Not Glutamate, in Enterally Fed Human Preterm Neonates CHRIS TOMLINSON, MAHROUKH RAFII, MICHAEL SGRO, RONALD O. BALL, AND PAUL PENCHARZ Department of Paediatrics [C.T., M.S., P.P.], Research Institute [C.T., M.R., P.P.], The Hospital for Sick Children, Toronto, Ontario M5G1X8, Canada; Department of Nutritional Sciences [C.T., M.S., P.P.], University of Toronto, Toronto, Ontario M5S3E2, Canada; Department of Paediatrics [M.S.], St Michael’s Hospital, Toronto, Ontario M5B1W8, Canada; Department of Agricultural, Food and Nutritional Science [R.O.B., P.P.], University of Alberta, Edmonton, Alberta T6G2P5, Canada ABSTRACT: In neonatal mammals, arginine is synthesized in the litis (NEC) (8) and pulmonary hypertension (9). Furthermore, enterocyte, with either proline or glutamate as the dietary precursor. arginine supplementation was shown to reduce the incidence We have shown several times in piglets that proline is the only of all stages of NEC in moderately at risk infants (10) and a precursor to arginine, although in vitro evidence supports glutamate single bolus infusion of i.v. arginine improved oxygenation in in this role. Because of this uncertainty, we performed a multitracer infants with pulmonary hypertension (11). Therefore, because stable isotope study to determine whether proline, glutamate, or both are dietary precursors for arginine in enterally fed human neonates. arginine is clearly important for metabolism in the neonate, it Labeled arginine (M ϩ 2), proline (M ϩ 1), and glutamate (M ϩ 3) is critical to understand the metabolic pathways involved in its were given enterally to 15 stable, growing preterm infants (GA at synthesis. -

Role of Cadaverine and Piperidine in the Formation of N-Nitrosopiperidine in Heated Cured Meat
ROLE OF CADAVERINE AND PIPERIDINE IN THE FORMATION OF N-NITROSOPIPERIDINE IN HEATED CURED MEAT Drabik-Markiewicz G.1, 2, De Mey E. 1, Impens S. 1, Kowalska T. 2, Vander Heyden Y. 3 and Paelinck H.1* 1Research Group for Technology and Quality of Animal Products, Catholic University College Gent, Technology Campus Gent, Department of Chemistry and Biochemistry, 1 Gebroeders Desmetstraat, 9000 Gent, Belgium 2University of Silesia, Institute of Chemistry, 9 Szkolna Street, 40-006 Katowice, Poland 3Analytical Chemistry and Pharmaceutical Technology, Center for Pharmaceutical Research (CePhaR), Vrije Universiteit Brussel (VUB), Laarbeeklaan 103, B-1090 Brussels, Belgium *Corresponding author (e-mail: [email protected]) Abstract — N-nitrosamines are carcinogenic compounds, which formation in meat products depends from different factors e.g., temperature, storage time, precursors and/or added sodium nitrite. Sodium nitrite is important for meat processing as curing agent. The aim of this study was to determine the role of cadaverine and piperidine on the formation of N-nitrosamines in heated cured meat products. Such experimental products were processed with different amounts of sodium nitrite ( 0 mg kg -1, 120 mg kg -1, 480 mg kg -1), 1000 mg kg -1 of cadaverine or 10 mg kg -1 of piperidine, and heated at 85°C, 120°C, 160°C or 220°C. Experimental evidence was produced using gas chromatography in combination with Thermal Energy Analyzer (GC-TEA). The obtained analytical results were statistically evaluated by means of the Univariate Analysis of Variance (ANOVA) approach. In the current study only N-nitrosodimethylamine (NDMA) and N-nitrosopiperidine (NPIP) were detected. -

Article the Bee Hemolymph Metabolome: a Window Into the Impact of Viruses on Bumble Bees
Article The Bee Hemolymph Metabolome: A Window into the Impact of Viruses on Bumble Bees Luoluo Wang 1,2, Lieven Van Meulebroek 3, Lynn Vanhaecke 3, Guy Smagghe 2 and Ivan Meeus 2,* 1 Guangdong Provincial Key Laboratory of Insect Developmental Biology and Applied Technology, Institute of Insect Science and Technology, School of Life Sciences, South China Normal University, Guangzhou, China; [email protected] 2 Department of Plants and Crops, Faculty of Bioscience Engineering, Ghent University, Ghent, Belgium; [email protected], [email protected] 3 Laboratory of Chemical Analysis, Department of Veterinary Public Health and Food Safety, Faculty of Vet- erinary Medicine, Ghent University, Merelbeke, Belgium; [email protected]; [email protected] * Correspondence: [email protected] Selection of the targeted biomarker set: In total we identified 76 metabolites, including 28 amino acids (37%), 11 carbohy- drates (14%), 11 carboxylic acids, 2 TCA intermediates, 4 polyamines, 4 nucleic acids, and 16 compounds from other chemical classes (Table S1). We selected biologically-relevant biomarker candidates based on a three step approach: (1) their expression profile in stand- ardized bees and its relation with viral presence, (2) pathways analysis on significant me- tabolites; and (3) a literature search to identify potential viral specific signatures. Step (1) and (2), pathways analysis on significant metabolites We performed two-way ANOVA with Tukey HSD tests for post-hoc comparisons and used significant metabolites for metabolic pathway analysis using the web-based Citation: Wang, L.L.; Van platform MetaboAnalyst (http://www.metaboanalyst.ca/) in order to get insights in the Meulebroek, L.; Vanhaecke.