Microbial Community Changes During a Toxic Cyanobacterial Bloom in an Alkaline Hungarian Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide
antioxidants Review Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide Ivan Kushkevych 1,* , Veronika Bosáková 1,2 , Monika Vítˇezová 1 and Simon K.-M. R. Rittmann 3,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] (V.B.); [email protected] (M.V.) 2 Department of Biology, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3 Archaea Physiology & Biotechnology Group, Department of Functional and Evolutionary Ecology, Universität Wien, 1090 Vienna, Austria * Correspondence: [email protected] (I.K.); [email protected] (S.K.-M.R.R.); Tel.: +420-549-495-315 (I.K.); +431-427-776-513 (S.K.-M.R.R.) Abstract: Hydrogen sulfide is a toxic compound that can affect various groups of water microorgan- isms. Photolithotrophic sulfur bacteria including Chromatiaceae and Chlorobiaceae are able to convert inorganic substrate (hydrogen sulfide and carbon dioxide) into organic matter deriving energy from photosynthesis. This process takes place in the absence of molecular oxygen and is referred to as anoxygenic photosynthesis, in which exogenous electron donors are needed. These donors may be reduced sulfur compounds such as hydrogen sulfide. This paper deals with the description of this metabolic process, representatives of the above-mentioned families, and discusses the possibility using anoxygenic phototrophic microorganisms for the detoxification of toxic hydrogen sulfide. Moreover, their general characteristics, morphology, metabolism, and taxonomy are described as Citation: Kushkevych, I.; Bosáková, well as the conditions for isolation and cultivation of these microorganisms will be presented. V.; Vítˇezová,M.; Rittmann, S.K.-M.R. -

Limits of Life on Earth Some Archaea and Bacteria
Limits of life on Earth Thermophiles Temperatures up to ~55C are common, but T > 55C is Some archaea and bacteria (extremophiles) can live in associated usually with geothermal features (hot springs, environments that we would consider inhospitable to volcanic activity etc) life (heat, cold, acidity, high pressure etc) Thermophiles are organisms that can successfully live Distinguish between growth and survival: many organisms can survive intervals of harsh conditions but could not at high temperatures live permanently in such conditions (e.g. seeds, spores) Best studied extremophiles: may be relevant to the Interest: origin of life. Very hot environments tolerable for life do not seem to exist elsewhere in the Solar System • analogs for extraterrestrial environments • `extreme’ conditions may have been more common on the early Earth - origin of life? • some unusual environments (e.g. subterranean) are very widespread Extraterrestrial Life: Spring 2008 Extraterrestrial Life: Spring 2008 Grand Prismatic Spring, Yellowstone National Park Hydrothermal vents: high pressure in the deep ocean allows liquid water Colors on the edge of the at T >> 100C spring are caused by different colonies of thermophilic Vents emit superheated water (300C or cyanobacteria and algae more) that is rich in minerals Hottest water is lifeless, but `cooler’ ~50 species of such thermophiles - mostly archae with some margins support array of thermophiles: cyanobacteria and anaerobic photosynthetic bacteria oxidize sulphur, manganese, grow on methane + carbon monoxide etc… Sulfolobus: optimum T ~ 80C, minimum 60C, maximum 90C, also prefer a moderately acidic pH. Live by oxidizing sulfur Known examples can grow (i.e. multiply) at temperatures which is abundant near hot springs. -

The Intestinal Protozoa
The Intestinal Protozoa A. Introduction 1. The Phylum Protozoa is classified into four major subdivisions according to the methods of locomotion and reproduction. a. The amoebae (Superclass Sarcodina, Class Rhizopodea move by means of pseudopodia and reproduce exclusively by asexual binary division. b. The flagellates (Superclass Mastigophora, Class Zoomasitgophorea) typically move by long, whiplike flagella and reproduce by binary fission. c. The ciliates (Subphylum Ciliophora, Class Ciliata) are propelled by rows of cilia that beat with a synchronized wavelike motion. d. The sporozoans (Subphylum Sporozoa) lack specialized organelles of motility but have a unique type of life cycle, alternating between sexual and asexual reproductive cycles (alternation of generations). e. Number of species - there are about 45,000 protozoan species; around 8000 are parasitic, and around 25 species are important to humans. 2. Diagnosis - must learn to differentiate between the harmless and the medically important. This is most often based upon the morphology of respective organisms. 3. Transmission - mostly person-to-person, via fecal-oral route; fecally contaminated food or water important (organisms remain viable for around 30 days in cool moist environment with few bacteria; other means of transmission include sexual, insects, animals (zoonoses). B. Structures 1. trophozoite - the motile vegetative stage; multiplies via binary fission; colonizes host. 2. cyst - the inactive, non-motile, infective stage; survives the environment due to the presence of a cyst wall. 3. nuclear structure - important in the identification of organisms and species differentiation. 4. diagnostic features a. size - helpful in identifying organisms; must have calibrated objectives on the microscope in order to measure accurately. -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

Bacterial Community Composition of Size-Fractioned Aggregates Within the Phycosphere of Cyanobacterial Blooms in a Eutrophic Freshwater Lake
Bacterial Community Composition of Size-Fractioned Aggregates within the Phycosphere of Cyanobacterial Blooms in a Eutrophic Freshwater Lake Haiyuan Cai1, Helong Jiang1*, Lee R. Krumholz2, Zhen Yang1 1 State Key Laboratory of Lake Science and Environment, Nanjing Institute of Geography and Limnology, Chinese Academy of Sciences, Nanjing, China, 2 Department of Botany and Microbiology, University of Oklahoma, Norman, Oklahoma, United States of America Abstract Bacterial community composition of different sized aggregates within the Microcystis cyanobacterial phycosphere were determined during summer and fall in Lake Taihu, a eutrophic lake in eastern China. Bloom samples taken in August and September represent healthy bloom biomass, whereas samples from October represent decomposing bloom biomass. To improve our understanding of the complex interior structure in the phycosphere, bloom samples were separated into large (.100 mm), medium (10–100 mm) and small (0.2–10 mm) size aggregates. Species richness and library coverage indicated that pyrosequencing recovered a large bacterial diversity. The community of each size aggregate was highly organized, indicating highly specific conditions within the Microcystis phycosphere. While the communities of medium and small-size aggregates clustered together in August and September samples, large- and medium-size aggregate communities in the October sample were grouped together and distinct from small-size aggregate community. Pronounced changes in the absolute and relative percentages of the dominant genus from the two most important phyla Proteobacteria and Bacteroidetes were observed among the various size aggregates. Bacterial species on large and small-size aggregates likely have the ability to degrade high and low molecular weight compounds, respectively. Thus, there exists a spatial differentiation of bacterial taxa within the phycosphere, possibly operating in sequence and synergy to catalyze the turnover of complex organic matters. -

Algae and Cyanobacteria in Coastal and Estuarine Waters
CHAPTER 7 Algae and cyanobacteria in coastal and estuarine waters n coastal and estuarine waters, algae range from single-celled forms to the seaweeds. ICyanobacteria are organisms with some characteristics of bacteria and some of algae. They are similar in size to the unicellular algae and, unlike other bacteria, contain blue-green or green pigments and are able to perform photosynthesis; thus, they are also termed blue-green algae. Algal blooms in the sea have occurred throughout recorded history but have been increasing during recent decades (Anderson, 1989; Smayda, 1989a; Hallegraeff, 1993). In several areas (e.g., the Baltic and North seas, the Adriatic Sea, Japanese coastal waters and the Gulf of Mexico), algal blooms are a recurring phe- nomenon. The increased frequency of occurrence has accompanied nutrient enrich- ment of coastal waters on a global scale (Smayda, 1989b). Blooms of non-toxic phytoplankton species and mass occurrences of macro-algae can affect the amenity value of recreational waters due to reduced transparency, dis- coloured water and scum formation. Furthermore, bloom degradation can be accom- panied by unpleasant odours, resulting in aesthetic problems (see chapter 9). Several human diseases have been reported to be associated with many toxic species of dinoflagellates, diatoms, nanoflagellates and cyanobacteria that occur in the marine environment (CDC, 1997). The effects of these algae on humans are due to some of their constituents, principally algal toxins. Marine algal toxins become a problem primarily because they may concentrate in shellfish and fish that are subsequently eaten by humans (CDR, 1991; Lehane, 2000), causing syndromes known as para- lytic shellfish poisoning (PSP), diarrhetic shellfish poisoning (DSP), amnesic shellfish poisoning (ASP), neurotoxic shellfish poisoning (NSP) and ciguatera fish poisoning (CFP). -
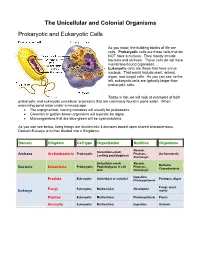
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

About Cyanobacteria BACKGROUND Cyanobacteria Are Single-Celled Organisms That Live in Fresh, Brackish, and Marine Water
About Cyanobacteria BACKGROUND Cyanobacteria are single-celled organisms that live in fresh, brackish, and marine water. They use sunlight to make their own food. In warm, nutrient-rich environments, microscopic cyanobacteria can grow quickly, creating blooms that spread across the water’s surface and may become visible. Because of the color, texture, and location of these blooms, the common name for cyanobacteria is blue-green algae. However, cyanobacteria are related more closely to bacteria than to algae. Cyanobacteria are found worldwide, from Brazil to China, Australia to the United States. In warmer climates, these organisms can grow year-round. Scientists have called cyanobacteria the origin of plants, and have credited cyanobacteria with providing nitrogen fertilizer for rice and beans. But blooms of cyanobacteria are not always helpful. When these blooms become harmful to the environment, animals, and humans, scientists call them cyanobacterial harmful algal blooms (CyanoHABs). Freshwater CyanoHABs can use up the oxygen and block the sunlight that other organisms need to live. They also can produce powerful toxins that affect the brain and liver of animals and humans. Because of concerns about CyanoHABs, which can grow in drinking water and recreational water, the U.S. Environmental Protection Agency (EPA) has added cyanobacteria to its Drinking Water Contaminant Candidate List. This list identifies organisms and toxins that EPA considers to be priorities for investigation. ASSESSING THE IMPACT ON PUBLIC HEALTH Reports of poisonings associated with CyanoHABs date back to the late 1800s. Anecdotal evidence and data from laboratory animal research suggest that cyanobacterial toxins can cause a range of adverse human health effects, yet few studies have explored the links between CyanoHABs and human health. -

Zooming in on the Phycosphere: the Ecological Interface for Phytoplankton–Bacteria Relationships Justin R
REVIEW ARTICLE PUBLISHED: 30 MAY 2017 | VOLUME: 2 | ARTICLE NUMBER: 17065 Zooming in on the phycosphere: the ecological interface for phytoplankton–bacteria relationships Justin R. Seymour1*, Shady A. Amin2,3, Jean-Baptiste Raina1 and Roman Stocker4 By controlling nutrient cycling and biomass production at the base of the food web, interactions between phytoplankton and bacteria represent a fundamental ecological relationship in aquatic environments. Although typically studied over large spa- tiotemporal scales, emerging evidence indicates that this relationship is often governed by microscale interactions played out within the region immediately surrounding individual phytoplankton cells. This microenvironment, known as the phycosphere, is the planktonic analogue of the rhizosphere in plants. The exchange of metabolites and infochemicals at this interface governs phytoplankton–bacteria relationships, which span mutualism, commensalism, antagonism, parasitism and competition. The importance of the phycosphere has been postulated for four decades, yet only recently have new technological and conceptual frameworks made it possible to start teasing apart the complex nature of this unique microbial habitat. It has subsequently become apparent that the chemical exchanges and ecological interactions between phytoplankton and bacteria are far more sophisticated than previously thought and often require close proximity of the two partners, which is facilitated by bacterial col- onization of the phycosphere. It is also becoming increasingly clear that while interactions taking place within the phycosphere occur at the scale of individual microorganisms, they exert an ecosystem-scale influence on fundamental processes including nutrient provision and regeneration, primary production, toxin biosynthesis and biogeochemical cycling. Here we review the fundamental physical, chemical and ecological features of the phycosphere, with the goal of delivering a fresh perspective on the nature and importance of phytoplankton–bacteria interactions in aquatic ecosystems. -

Molecular Mechanisms Involved in Prokaryotic Cycling of Labile Dissolved Organic Matter in the Sea
Molecular mechanisms involved in prokaryotic cycling of labile dissolved organic matter in the sea Linnaeus University Dissertations No 412/2021 MOLECULAR MECHANISMS INVOLVED IN PROKARYOTIC CYCLING OF LABILE DISSOLVED ORGANIC MATTER IN THE SEA BENJAMIN PONTILLER LINNAEUS UNIVERSITY PRESS Molecular mechanisms involved in prokaryotic cycling of labile dissolved organic matter in the sea Doctoral Dissertation, Department of Biology and Environmental Science, Linnaeus University, Kalmar, 2021 ISBN: 978-91-89283-65-7 (print), 978-91-89283-66-4 (pdf) Published by: Linnaeus University Press, 351 95 Växjö Printed by: Holmbergs, 2021 Abstract Pontiller, Benjamin (2021). Molecular mechanisms involved in prokaryotic cycling of labile dissolved organic matter in the sea, Linnaeus University Dissertations No 412/2021, ISBN: 978-91-89283-65-7 (print), 978-91-89283-66-4 (pdf) . Roughly half of the global primary production originates from microscopic phytoplankton in marine ecosystems, converting carbon dioxide into organic matter. This organic matter pool consists of a myriad of compounds that fuel heterotrophic bacterioplankton. However, knowledge of the molecular mechanisms – particularly the metabolic pathways involved in the degradation and utilization of dissolved organic matter (DOM) – and transcriptional dynamics over spatiotemporal gradients are still scarce. Therefore, we studied the molecular mechanisms of bacterioplankton communities, including archaea, involved in the cycling of DOM, over different spatiotemporal scales in experiments and through field observations. In seawater experiments, we found a divergence of bacterioplankton transcriptional responses to different organic matter compound classes (carbohydrates, nucleic acids, and proteins) and condensation states (monomers or polymers). These responses were associated with distinct bacterial taxa, suggesting pronounced functional partitioning of these compounds in the Sea. -

Metabarcoding for Bacterial Diversity Assessment: Looking Inside Didymosphenia Geminata Mats in Patagonian Aquatic Ecosystems
Aquatic Invasions (2021) Volume 16, Issue 1: 43–61 Special Issue: Proceedings of the 21st International Conference on Aquatic Invasive Species Guest editors: Sarah Bailey, Bonnie Holmes and Oscar Casas-Monroy CORRECTED PROOF Research Article Metabarcoding for bacterial diversity assessment: looking inside Didymosphenia geminata mats in Patagonian aquatic ecosystems Ana Victoria Suescún1,*, Karla Martinez-Cruz2, Maialen Barret3 and Leyla Cárdenas1,4 1Instituto de Ciencias Ambientales y Evolutivas, Facultad de Ciencias, Universidad Austral de Chile, Casilla 567, Valdivia, Chile 2Environmental Biogeochemistry in Extreme Ecosystems Laboratory, Universidad de Magallanes, Punta Arenas, Chile 3Laboratory of Functional Ecology and Environment, Université de Toulouse, CNRS, Toulouse, France 4Centro FONDAP de Investigación en Dinámica de Ecosistemas Marinos de Altas Latitudes (IDEAL), Chile Author e-mails: [email protected] (AVS), [email protected] (KM), [email protected] (MB), [email protected] (LC) *Corresponding author Co-Editors’ Note: This study was contributed in relation to the 21st International Conference Abstract on Aquatic Invasive Species held in Montreal, Canada, October 27–31, 2019 (http://www.icais. The number of organisms that spread and invade new habitats has increased in recent org/html/previous21.html). This conference has decades as a result of drastic environmental changes such as climate change and provided a venue for the exchange of anthropogenic activities. Microbial species invasions occur worldwide in terrestrial information on various aspects of aquatic and aquatic systems and represent an emerging challenge to our understanding of invasive species since its inception in 1990. The conference continues to provide an the interplay between biodiversity and ecosystem functioning. Due to the difficulty opportunity for dialog between academia, of detecting and evaluating non-indigenous microorganisms, little is known about industry and environmental regulators. -

Harmful Cyanobacteria Blooms and Their Toxins In
Harmful cyanobacteria blooms and their toxins in Clear Lake and the Sacramento-San Joaquin Delta (California) 10-058-150 Surface Water Ambient Monitoring Program (SWAMP) Prepared for: Central Valley Regional Water Quality Control Board 11020 Sun Center Drive, Suite 200 Rancho Cordova, CA 95670 Prepared by: Cécile Mioni (Project Director) & Raphael Kudela (Project co-Director) University of California, Santa Cruz - Institute of Marine Sciences Dolores Baxa (Project co-Director) University of California, Davis – School of Veterinary Medicine Contract manager: Meghan Sullivan Central Valley Regional Water Quality Control Board _________________ With technical contributions by: Kendra Hayashi (Project manager), UCSC Thomas Smythe (Field Officer) and Chris White, Lake County Water Resources Scott Waller (Field Officer) and Brianne Sakata, EMP/DWR Tomo Kurobe (Molecular Biologist), UCD David Crane (Toxicology), DFG-WPCL Kim Ward, SWRCB/DWQ Lenny Grimaldo (Assistance for Statistic Analyses), Bureau of Reclamation Peter Raimondi (Assistance for Statistic Analyses), UCSC Karen Tait, Lake County Health Office Abstract Harmful cyanobacteria and their toxins are growing contaminants of concern. Noxious toxins produced by HC, collectively referred as cyanotoxins, reduce the water quality and may impact the supply of clean water for drinking as well as the water quality which directly impacts the livelihood of other species including several endangered species. USEPA recently (May 29, 2008) made the decision to add microcystin toxins as an additional cause of impairment for the Klamath River, CA. However, harmful cyanobacteria are some of the less studied causes of impairment in California water bodies and their distribution, abundance and dynamics, as well as the conditions promoting their proliferation and toxin production are not well characterized.