The Bohr Effect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Physiology an Integrated Approach
Gas Exchange and Transport Gas Exchange in the Lungs and Tissues 18 Lower Alveolar P Decreases Oxygen Uptake O2 Diff usion Problems Cause Hypoxia Gas Solubility Aff ects Diff usion Gas Transport in the Blood Hemoglobin Binds to Oxygen Oxygen Binding Obeys the Law of Mass Action Hemoglobin Transports Most Oxygen to the Tissues P Determines Oxygen-Hb Binding O2 Oxygen Binding Is Expressed As a Percentage Several Factors Aff ect Oxygen-Hb Binding Carbon Dioxide Is Transported in Three Ways Regulation of Ventilation Neurons in the Medulla Control Breathing Carbon Dioxide, Oxygen, and pH Infl uence Ventilation Protective Refl exes Guard the Lungs Higher Brain Centers Aff ect Patterns of Ventilation The successful ascent of Everest without supplementary oxygen is one of the great sagas of the 20th century. — John B. West, Climbing with O’s , NOVA Online (www.pbs.org) Background Basics Exchange epithelia pH and buff ers Law of mass action Cerebrospinal fl uid Simple diff usion Autonomic and somatic motor neurons Structure of the brain stem Red blood cells and Giant liposomes hemoglobin of pulmonary Blood-brain barrier surfactant (40X) From Chapter 18 of Human Physiology: An Integrated Approach, Sixth Edition. Dee Unglaub Silverthorn. Copyright © 2013 by Pearson Education, Inc. All rights reserved. 633 Gas Exchange and Transport he book Into Thin Air by Jon Krakauer chronicles an ill- RUNNING PROBLEM fated trek to the top of Mt. Everest. To reach the summit of Mt. Everest, climbers must pass through the “death zone” T High Altitude located at about 8000 meters (over 26,000 ft ). Of the thousands of people who have attempted the summit, only about 2000 have been In 1981 a group of 20 physiologists, physicians, and successful, and more than 185 have died. -

Risk Assessment of Recirculation Systems in Salmonid Hatcheries
Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Risk Assessment of Recirculation Systems in Salmonid Hatcheries Opinion of the Panel on Animal Health and Welfare of the Norwegian Scientific Committee for Food Safety Date: 10.01.12 Doc. no.: 09-808-Final ISBN: 978-82-8259-048-8 VKM Report 2012: 01 1 Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Risk Assessment of Recirculation Systems in Salmonid Hatcheries Brit Hjeltnes (chair of ad hoc group) Grete Bæverfjord Ulf Erikson Stein Mortensen Trond Rosten Peter Østergård 2 Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Contributors Persons working for VKM, either as appointed members of the Committee or as ad hoc experts, do this by virtue of their scientific expertise, not as representatives for their employers. The Civil Services Act instructions on legal competence apply for all work prepared by VKM. Acknowledgements The Norwegian Scientific Committee for Food Safety (Vitenskapskomiteen for mattrygghet, VKM) has appointed an ad hoc group consisting of both VKM members and external experts to answer the request from the Norwegian Food Safety Authority. The members of the ad hoc group are acknowledged for their valuable work on this opinion. The members of the ad hoc group are: VKM members Brit Hjeltnes (Chair), Panel on Animal Health and Welfare Ulf Erikson, Panel on Animal Health and Welfare Stein Mortensen, Panel on Animal Health and Welfare External experts Grete Bæverfjord, Nofima Marin, Sunndalsøra Trond Rosten, SINTEF Fisheries and Aquaculture Peter Østergård, Sp/F Aquamed, Faroe Islands Other contributors to the assessment are Frode Mathisen, Anders Fjellheim and Brit Tørud. -

Respiratory Physiology
Physiology Unit 4 RESPIRATORY PHYSIOLOGY Respiraon • External respiraon – ven3laon – gas exchange • Internal respiraon – cellular respiraon – gas exchange • Respiratory Cycle – Inspiraon • Moving atmospheric air into the lungs – Expiraon • Moving air out of the lungs Lungs vs. Balloons • A lung is similar to a balloon in that it resists stretch, tending to collapse almost totally unless held inflated by a pressure difference between its inside and outside • Lungs and the chest have elas3c proper3es Lung Compliance • Compliance – Elas3city – Tendency to recoil – Tendency of an elas3c structure to oppose stretching or distor3on * Resists distension • Surface tension * Resists distension - Surfactant • Reduces surface tension • Increases compliance (makes them easier to stretch) Airway Resistance F = ΔP/R • Same variables that affect resistance in blood vessels – Tube length, tube radius, fric3on – Tube radius most important factor • Airway resistance is so small that small pressure differences produce large volumes of air flow – Average atmosphere-to-alveoli pressure difference is 1 mmHg, but 500 mL of air is moved (%dal volume) – Low pressure and low resistance • Pulmonary 1/10th of systemic vascular resistance Ven3laon • Exchange of air between atmosphere and alveoli • Atmospheric air pressure is 760 mmHg at sea level • Air moves by bulk flow – F = ΔP/R – F = (Palv – Patm)/R Boyle’s Law • Boyle’s law = (P/V) • Pressure of a given quan3ty of gas is inversely propor3onal to volume • An increase in the volume of the container (lungs) decreases the pressure of the gas (air) Ven3laon Mechanics • Lung volume depends on: 1. Transpulmonary pressure (Ptp) • Inside to outside of the lung • Ptp = Palv – Pip • The force that keeps the lungs inflated • Transmural pressure – Across the wall 2. -

Chapter 16 I. the Respiratory System Respiratory System Respiration Gas Exchange in Lungs Alveoli
10/24/11 I. The Respiratory System Chapter 16 Respiratory Physiology Lecture PowerPoint Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Respiratory System Respiration • Includes: • Divided into: – Ventilation (breathing) – Gas exchange between blood and lungs and – Respiratory zone: site of gas exchange between blood and tissues – Oxygen utilization by tissues to make ATP – Conduction zone: gets air to the respiratory • Ventilation and gas exchange in lungs = zone external respiration • Oxygen utilization and gas exchange in tissues = internal respiration Gas Exchange in Lungs Alveoli • Occurs via diffusion • Air sacs in the lungs where gas exchange occurs • O2 concentration is higher in the lungs than in the blood, so O2 diffuses into blood. • 300 million of them – Provide large surface area (760 square feet) to increase diffusion rate • CO2 concentration in the blood is higher than in the lungs, so CO2 diffuses out of blood. 1 10/24/11 Alveoli Alveoli Capillary within alveolar wall Bronchiole and alveoli Alveolar Cells Conducting Zone • Air travels down the nasal cavity Pharynx • Type I: 95−97% total surface area where Larynx gas exchange occurs Trachea Right and left primary bronchi • Type II: secrete pulmonary surfactant and Secondary bronchi reabsorb sodium and water, preventing Tertiary bronchi (more branching) Terminal bronchioles fluid buildup Respiratory zone (respiratory bronchioles Terminal alveolar sacs Respiratory Structures Trachea and Respiratory Bronchi 2 10/24/11 Functions of Conducting Zone Functions of Conducting Zone • Transports air to the lungs • Warms, humidifies, filters, and cleans the air – Mucus traps small particles, and cilia move it away from the lungs. • Voice production in the larynx as air passes over the vocal folds Thoracic Cavity Thoracic Cavity Cross Section • Contains the heart, trachea, esophagus, and thymus within the central mediastinum • The lungs fill the rest of the cavity. -

Respiratory System.Pdf
Respiratory System Respiratory System - Overview: Assists in the detection Protects system of odorants Respiratory (debris / pathogens / dessication) System 5 3 4 Produces sound (vocalization) Provides surface area for gas exchange (between air / blood) 1 2 For the body to survive, there must be a constant supply of Moves air to / from gas O2 and a constant exchange surface disposal of CO 2 Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Table 19.1 Respiratory System Respiratory System Functional Anatomy: Functional Anatomy: Trachea Epiglottis Naming of pathways: • > 1 mm diameter = bronchus Upper Respiratory • Conduction of air • < 1 mm diameter = bronchiole System • Gas exchange Primary • < 0.5 mm diameter = terminal bronchiole Bronchus • Filters / warms / humidifies Lower Respiratory Bronchi System incoming air bifurcation (23 orders) 1) External nares 5) Larynx 2) Nasal cavity • Provide open airway Green = Conducting zone • Resonance chamber • channel air / food Purple = Respiratory zone 3) Uvula • voice production (link) 4) Pharynx 6) Trachea 7) Bronchial tree • Nasopharynx Bronchiole 8) Alveoli • Oropharynx Terminal Bronchiole Respiratory Bronchiole • Laryngopharynx Alveolus Martini et. al. (Fundamentals of Anatomy and Physiology, 7th ed.) – Figure 23.1 Martini et. al. (Fundamentals of Anatomy and Physiology, 7th ed.) – Figure 23.9 Respiratory System Respiratory System Functional Anatomy: Functional Anatomy: Respiratory Mucosa / Submucosa: How are inhaled debris / pathogens cleared from respiratory tract? Near Near trachea alveoli Nasal Cavity: Epithelium: Particles > 10 µm Pseudostratified Simple columnar cuboidal Conducting Zone: Particles 5 – 10 µm Cilia No cilia Respiratory Zone: Mucus Escalator Particles 1 – 5 µm Mucosa: Lamina Propria (areolar tissue layer): Mucous membrane (epithelium / areolar tissue) smooth smooth muscle muscle Mucous No glands mucous glands Cartilage: Rings Plates / none Macrophages Martini et. -

Pulmonary Ventilation
CHAPTER 2 Pulmonary Ventilation © IT Stock/Polka Dot/ inkstock Chapter Objectives By studying this chapter, you should be able to do Name two things that cause pleural pressure the following: to decrease. 8. Describe the mechanics of ventilation with 1. Identify the basic structures of the conducting respect to the changes in pulmonary pressures. and respiratory zones of the ventilation system. 9. Identify the muscles involved in inspiration and 2. Explain the role of minute ventilation and its expiration at rest. relationship to the function of the heart in the 10. Describe the partial pressures of oxygen and production of energy at the tissues. carbon dioxide in the alveoli, lung capillaries, 3. Identify the diff erent ways in which carbon tissue capillaries, and tissues. dioxide is transported from the tissues to the 11. Describe how carbon dioxide is transported in lungs. the blood. 4. Explain the respiratory advantage of breathing 12. Explain the significance of the oxygen– depth versus rate during a treadmill exercise. hemoglobin dissociation curve. 5. Describe the composition of ambient air and 13. Discuss the eff ects of decreasing pH, increasing alveolar air and the pressure changes in the pleu- temperature, and increasing 2,3-diphosphoglyc- ral and pulmonary spaces. erate on the HbO dissociation curve. 6. Diagram the three ways in which carbon dioxide 2 14. Distinguish between and explain external res- is transported in the venous blood to the lungs. piration and internal respiration. 7. Defi ne pleural pressure. What happens to alve- olar volume when pleural pressure decreases? Chapter Outline Pulmonary Structure and Function Pulmonary Volumes and Capacities Anatomy of Ventilation Lung Volumes and Capacities Lungs Pulmonary Ventilation Mechanics of Ventilation Minute Ventilation Inspiration Alveolar Ventilation Expiration Pressure Changes Copyright ©2014 Jones & Bartlett Learning, LLC, an Ascend Learning Company Content not final. -

Pulmonary Function Tests
Pulmonary Function Tests • Forced Vital Capacity (FVC) measures the amt of gas expelled when one takes a deep breath and then forcefully exhales maximally and rapidly. • Forced Expiratory Volume (FEV) determines the amt of air expelled during specific time intervals of the FVC test. – For example, the volume exhaled during the 1st second is the FEV1. People w/ healthy lungs can exhale about 80% of the FVC in the 1st second. Obstructive Disease • Difficult to get air out of the lungs • Obstruct expiration • Examples: – emphysema – chronic bronchitis – asthma. Restrictive Disease • Difficult to get air in to the lungs • ―Restrict‖ inspiration • Examples: – intersitial fibrosis – muscular diseases – chestwall deformities. Lung Capacity and Disease— Summary • Obstructive Disease: – Decreased VC – Increased TLC, RV, FRC. • Restrictive Disease: – Decreased VC – Decreased TLC, RV, FRC. Forced Vital Capacity FEV1.0 / FVC Ratio Small Airways Disease FEF25-75 Flow -Volume Curves Peak Flow Dead Space Anatomical dead space – volume of the conducting respiratory passages (150 ml) Alveolar dead space – alveoli that cease to act in gas exchange due to collapse or obstruction Total dead space – sum of alveolar and anatomical dead spaces Alveolar Ventilation • Alveolar ventilation rate (AVR) – measures the flow of fresh gases into and out of the alveoli during a particular time AVR = frequency X (TV – dead space) (ml/min) (breaths/min) (ml/breath) • Slow, deep breathing increases AVR and rapid, shallow breathing decreases AVR Gas Laws Govern O2 and CO2 Saturation of Blood 1. Dalton’s Law - total pressure of a mixture of gases = sum of pressures of individual gases in the mix • pressure of a single gas in the mixture = partial pressure 2. -
Gas Transport Graphics Are Used with Permission Of: Adam.Com ( Benjamin Cummings Publishing Co ( Page 1
Gas Transport Graphics are used with permission of: adam.com (http://www.adam.com/) Benjamin Cummings Publishing Co (http://www.awl.com/bc) Page 1. Introduction • The blood transports oxygen and carbon dioxide between the lungs and other tissues throughout the body. • These gases are carried in several different forms: 1. dissolved in the plasma 2. chemically combined with hemoglobin 3. converted into a different molecule Page 2. Goals • To explore how oxygen is transported in the blood. • To explore how carbon dioxide is transported in the blood. • To understand the effect of variables, such as PO2 and PCO2, on oxygen and carbon dioxide transport. Page 3. Oxygen Transport • Transport of oxygen during external respiration: • With its low solubility, only approximately 1.5% of the oxygen is transported dissolved i n plasma. • The remaining 98.5% diffuses into red blood cells and chemically combines with hemoglobin. • Label this diagram: Page 4. Hemoglobin • Within each red blood cell, there are approximately 250 million hemoglobin molecules. • Each hemoglobin molecule consists of: 1. A globin portion composed of 4 polypeptide chains. 2. Four iron-containing pigments called heme groups. • Each hemoglobin molecule can transport up to 4 oxygen molecules because each iron atom can bind one oxygen molecule. • When 4 oxygen molecules are bound to hemoglobin, it is 100% saturated; when there are fewer, it is partially saturated. • Oxygen binding occurs in response to the high partial pressure of oxygen in the lungs. • When hemoglobin binds with oxygen, it is called oxyhemoglobin. • When one oxygen binds to hemoglobin, the other oxygen molecules bind more readily. -

• Transport of Oxygen and Carbon Dioxide
Class: B.Sc. HONOURS ZOOLOGY, IV Sem Paper: Animal Physiology: LSS (Theory Class) Teacher’s name: Meenakshi Rana Date: 28th March, 2020 Time: 12:30 – 2:30 PM Transport of oxygen and carbon dioxide Carbon dioxide transport Under normal resting conditions, each 100 mL of deoxygenated blood contains the equivalent of 53 mL of gaseous CO2, which is transported in the blood in three main forms. 1. Dissolved CO2: The smallest percentage—about 7%—is dissolved in blood plasma. Upon reaching the lungs, it diffuses into alveolar air and is exhaled. 2. Carbamino compound: A somewhat higher percentage, about 23%, combines with the amino groups of amino acids and proteins in blood to form carbamino compounds. Because the most prevalent protein in blood is hemoglobin (inside red blood cells), most of the CO2 transported in this manner is bound to hemoglobin. The main CO2 binding sites are the terminal amino acids in the two alpha and two beta globin chains. Hemoglobin that has bound CO2 is termed carbaminohemoglobin (Hb–CO2). The formation of carbaminohemoglobin is greatly influenced by PCO2. For example, in tissue capillaries PCO2 is relatively high, which promotes formation of carbaminohemoglobin. But in pulmonary capillaries, PCO2 is relatively low, and the CO2 readily splits apart from globin and enters the alveoli by diffusion. 3. Bicarbonate ions: The greatest percentage of CO2—about 70%—is transported in blood plasma as bicarbonate ions (HCO3¯). As CO2 diffuses into systemic capillaries and enters red 1 blood cells, it reacts with water in the presence of the enzyme carbonic anhydrase (CA) to form + carbonic acid, which dissociates into H and HCO3¯: Thus, as blood picks up CO2, HCO3¯ accumulates inside RBCs. -

Respiratory Physiology
RESPIRATORY PHYSIOLOGY By EMOJEVWE, V. DEPARTMENT OF PHYSIOLOGY UNIVERSITY OF MEDICAL SCIENCES,ONDO 14-Jun-17 1 Objectives of this course Students should Know the meaning of respiration Explain how the intrapulmonary and intrapleural pressures vary during ventilation and relate these pressure changes to Boyle’s law. Define the terms compliance and elasticity, and explain now these lung properties affect ventilation. Discuss the significance of surface tension in lung mechanics, explain how the law of Laplace applies to lung function and describe the role of pulmonary surfactant. To Know the pulmonary function tests and their importance Pulmonary disease should have also been studied 11/17/2017 2 Objectives (continued) Explain how inspiration and expiration are accomplished in unforced breathing and describe the accessory respiratory muscles used in forced breathing. Describe the roles of the medulla, pons, and cerebral cortex in the regulation of breathing. Explain how chemoreceptors in the medulla and the peripheral chemoreceptors in the aortic and carotid bodies respond to changes in PC02, pH, and P02. 11/17/2017 3 Objectives (continued) Describe the loading and unloading reactions and explain how the extent of these reactions is influenced by the P02 and affinity of HB for 02. Explain how oxygen transport is influenced by changes in blood pH, temperature, and explain the effect and physiological significance of 2,3-DPG on oxygen transport. Describe the hyperpnea of exercise and explain how the anaerobic threshold is 11/17/2017affected by endurance training. 4 Respiration Definition: It is the exchange of gases between the organism and its environment, utilization of O2 and production of CO2 by the organism. -

RESPIRATION- Notes I
RESPIRATION- Notes I By the end of this section, you will be able to LEARN: - Important topic • the principles of oxygen transport • Oxygen-hb dissociation curve • the structure of hemoglobin • the principles of carbon dioxide transport We know, the function of respiration is to provide oxygen for use by body cells during cellular respiration and to eliminate carbon dioxide, a waste product of cellular respiration, from the body. In order for the exchange of oxygen and carbon dioxide to occur, both gases must be transported between the external and internal respiration sites. Although carbon dioxide is more soluble than oxygen in blood, both gases require a specialized transport system for the majority of the gas molecules to be moved between the lungs and other tissues. Principles of oxygen transport • Oxygen is not very soluble in liquids. • A small amount of oxygen does dissolve in the blood and is transported in the bloodstream, but it is only about 1.5% of the total amount. • The majority of oxygen molecules are carried from the lungs to the body’s tissues by a specialized transport system, which relies on the erythrocyte—the red blood cell. • Erythrocytes contain a metalloprotein, hemoglobin, which serves to bind oxygen molecules to the erythrocyte ( Figure 1 ). • Heme is the portion of hemoglobin that contains iron, and it is heme that binds oxygen. One hemoglobin molecule contains iron-containing Heme molecules, and because of this, each hemoglobin molecule is capable of carrying up to four molecules of oxygen. • As oxygen diffuses across the respiratory membrane from the alveolus to the capillary, it also diffuses into the red blood cell and is bound by hemoglobin. -
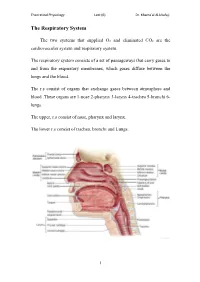
The Respiratory System
Theoretical Physiology Lect (6) Dr. Khama’al Al-khafaji The Respiratory System The two systems that supplied O2 and eliminated CO2 are the cardiovascular system and respiratory system. The respiratory system consists of a set of passageways that carry gases to and from the respiratory membranes, which gases diffuse between the lungs and the blood. The r.s consist of organs that exchange gases between atmosphere and blood .These organs are 1-nose 2-pharynx 3-larynx 4-trachea 5-bronchi 6- lungs. The upper, r.s consist of nose, pharynx and larynx. The lower r.s consist of trachea, bronchi and Lungs. 1 Theoretical Physiology Lect (6) Dr. Khama’al Al-khafaji Respiration: is the overall exchange of gases between the atmosphere, blood and cells. There are three basic processes involved in respiration:- 1-pulmonary ventilation (breathing): is the inspiration (in flow) and expiration (out flow) of air between the atmosphere and the lung. 2-External respiration: is the exchange of gases between the lungs and the blood. 3-Internal respiration: is the exchange of gases between the blood and the cells. A) Nose consist of - 1-External nares 2-Nasal cavity 3-Nasal septum 4-conchae 5-Internal nares. *Nasal cavity in the internal position contain vestibule *The conchae contain the olfactory receptors. *Functions of the structure, of the nose are:- 1-incoming air is wormed, humidified and filtered 2-olfactory stimuli is received 3-large hollow resonating chambers are provided for speech sound. B) Pharynx (throat): Is a muscular tube lined by mucous membrane and divided into:- 1-nasopharynx:-functions in respiration 2-oropharynx:-functions in digestion and respiration.