Gas Transport Graphics Are Used with Permission Of: Adam.Com ( Benjamin Cummings Publishing Co ( Page 1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Physiology an Integrated Approach
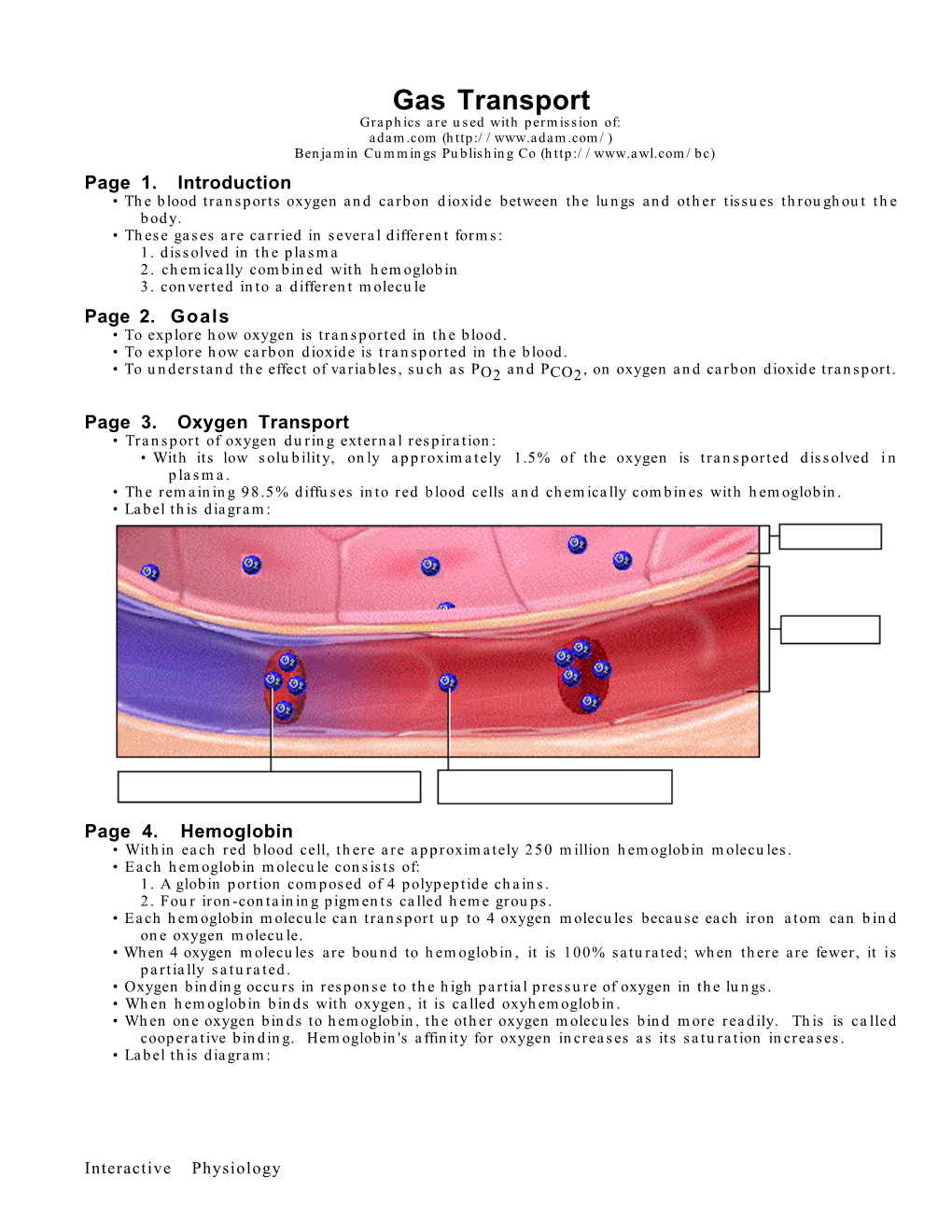
Gas Exchange and Transport Gas Exchange in the Lungs and Tissues 18 Lower Alveolar P Decreases Oxygen Uptake O2 Diff usion Problems Cause Hypoxia Gas Solubility Aff ects Diff usion Gas Transport in the Blood Hemoglobin Binds to Oxygen Oxygen Binding Obeys the Law of Mass Action Hemoglobin Transports Most Oxygen to the Tissues P Determines Oxygen-Hb Binding O2 Oxygen Binding Is Expressed As a Percentage Several Factors Aff ect Oxygen-Hb Binding Carbon Dioxide Is Transported in Three Ways Regulation of Ventilation Neurons in the Medulla Control Breathing Carbon Dioxide, Oxygen, and pH Infl uence Ventilation Protective Refl exes Guard the Lungs Higher Brain Centers Aff ect Patterns of Ventilation The successful ascent of Everest without supplementary oxygen is one of the great sagas of the 20th century. — John B. West, Climbing with O’s , NOVA Online (www.pbs.org) Background Basics Exchange epithelia pH and buff ers Law of mass action Cerebrospinal fl uid Simple diff usion Autonomic and somatic motor neurons Structure of the brain stem Red blood cells and Giant liposomes hemoglobin of pulmonary Blood-brain barrier surfactant (40X) From Chapter 18 of Human Physiology: An Integrated Approach, Sixth Edition. Dee Unglaub Silverthorn. Copyright © 2013 by Pearson Education, Inc. All rights reserved. 633 Gas Exchange and Transport he book Into Thin Air by Jon Krakauer chronicles an ill- RUNNING PROBLEM fated trek to the top of Mt. Everest. To reach the summit of Mt. Everest, climbers must pass through the “death zone” T High Altitude located at about 8000 meters (over 26,000 ft ). Of the thousands of people who have attempted the summit, only about 2000 have been In 1981 a group of 20 physiologists, physicians, and successful, and more than 185 have died. -

Hemoglobin : Its Protein of Molecular Weight 64,450 , in Human Beings It
Hemoglobin : its protein of molecular weight 64,450 , in human beings it is enclose in the RBC .if it were in plasma, some of it leaks through the capillary membrane into the tissue space or through the glomerular membrane of the kidney into the glomerular filtrate each time the blood passes through the capillaries , high free plasma concentration of Hb increased blood viscosity and osmotic pressure. So for Hb to remain in the bloodstream , it must exist in the RBCs ,its major function is to carry O2 to the tissue and also it transport CO2 from the tissues to the lungs Normal hemoglobin type: Hb A: Its normal adult Hb . Its molecule consist of four polypeptide chains ,2 alpha (α) chains (each of which contains 141 amino acids) and 2 beta chains (each of which contains 146 amino acids).thus Hb A is designated α2 and β2. Hb A is predominant type of Hb in adult (95- 97% of total Hb) . Hb A2 : in the normal adult about 25% of the total Hb is Hb A2 in which chain are replaced by delta chains and is designated 2 α 2δ2 . Each δ chain also contain 146 amino acid but 10 amino acid differ from those in the β chain . Hb F (Fetal Hb): it is the main Hb in fetus and new born . It is 2α 2γ,gamma(γ) chain also has 146 amino acid but 37 amino acid differ from those in β chain, Hb F is replaced gradually by adult Hb soon after birth, usually at about 6 months to one year of age, the normal adult Hb predominates . -

Metabolic Stable Isotope Fractionation
Photograph by author, Gina M.A. Carroll Metabolic Stable Isotope Fractionation: Biogeochemical Approaches to Diagnosing Sickle Cell and Thalassemia Anemia in the Archaeological Record MSc Thesis Faculty of Archaeology MSc Proefschrift Faculteit der Archaeologie Gina M.A. Carroll 1 Photograph by Gina. M.A. Carroll Taken with permission from the Municipal Museum of Écija, Spain April 2014 Gina M.A. Carroll Alberta, Canada Leiden, The Netherlands [email protected] 1 Metabolic Stable Isotope Fractionation: Biogeochemical Approaches to Diagnosing Sickle Cell and Thalassemia Anemia in the Archaeological Record. MSc Thesis MSc Proefschrift Gina M.A. Carroll Human Osteology and Funerary s1371266 Archaeology MSc Thesis Archaeology University of Leiden Faculty of Archaeology ARCH 1044WY Prof. Dr. Waters-Rist Leiden, The Netherlands & Prof. Dr. Inskip Leiden, 26 May 2015 Final Draft. 2 TABLE OF CONTENTS DEDICATIONS ...................................................................................................................... 9 ACKNOWLEDGEMENTS .................................................................................................. 10 CHAPTER 1 INTRODUCTION ....................................................................... 12-30 1. BRIEF HISTORY OF ARCHAEOLOGICAL RESEARCH ........................................ 13 1.1. The Anemias in Archaeology ....................................................... 14 1.2. The Application of Stable Isotopes in Palaeopathology ............... 18 2. HYPOTHESIS ................................................................................................ -

Published on May 14, 2008 As Doi: 10.1183/09031936.00126507 ERJ
ERJ Express. Published on May 14, 2008 as doi: 10.1183/09031936.00126507 ACCURACY AND RELIABILITY OF PULSE OXIMETRY AT DIFFERENT PaCO2 LEVELS Authors: Muñoz Xa,b,d , Torres Fc , Sampol Ga,d , Rios Jc , Martí Sa,d , Escrich Eb a) Servei de Pneumologia,Hospital Universitari Vall d’Hebron, Barcelona, Spain b) Departament de Biología Cel·lular, de Fisiologia i d’Immunologia, UAB, Barcelona, Spain c) Laboratorio de Bioestadística i Epidemiología (Universitat Autònoma de Barcelona); Servei de Farmacologia Clínica, IDIBAPS, (Hospital Clínic), Barcelona d) CIBER de Enfermedades Respiratorias (Ciberes) Correspondence to: Dr. Xavier Muñoz Servei de Pneumologia Hospital Vall d'Hebron Pº Vall d'Hebron, 119-129 08035 Barcelona Spain Telf: 00 34 93 2746157 Fax: 00 34 93 2746083 E-mail: [email protected] Short title: ACCURACY OF PULSE OXIMETRY AND PaCO2 LEVELS The first two authors have contributed equally to this study. Copyright 2008 by the European Respiratory Society. ABSTRACT Aim: To assess whether arterial carbon dioxide pressure (PaCO2) has an impact on agreement between oxygen saturation measured with pulse oximetry (SpO2) or arterial blood gas co- oximetry (SaO2). Methods: A study was performed on SaO2 and SpO2 determinations obtained simultaneously from 846 patients under assessment for long-term home oxygen therapy in a specialized outpatient clinic. Both measurements were taken with patients seated and breathing room air. Agreement between SaO2 and SpO2 results was analyzed by the Bland-Altman method and the Lin concordance coefficient. In addition, potential interactions of PaO2 or PaCO2 on agreement were analyzed by adjusted multivariate analysis. Results: At comparison of SaO2 and SpO2 results, the Bland-Altman technique yielded a bias (95% CI) of -1.24 (-6.86; 4.38) and -1.32 (-7.78; 5.15) when PaCO2 was higher than 48 mmHg or PaO2 lower than 54 mmHg, respectively. -

Risk Assessment of Recirculation Systems in Salmonid Hatcheries
Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Risk Assessment of Recirculation Systems in Salmonid Hatcheries Opinion of the Panel on Animal Health and Welfare of the Norwegian Scientific Committee for Food Safety Date: 10.01.12 Doc. no.: 09-808-Final ISBN: 978-82-8259-048-8 VKM Report 2012: 01 1 Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Risk Assessment of Recirculation Systems in Salmonid Hatcheries Brit Hjeltnes (chair of ad hoc group) Grete Bæverfjord Ulf Erikson Stein Mortensen Trond Rosten Peter Østergård 2 Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Contributors Persons working for VKM, either as appointed members of the Committee or as ad hoc experts, do this by virtue of their scientific expertise, not as representatives for their employers. The Civil Services Act instructions on legal competence apply for all work prepared by VKM. Acknowledgements The Norwegian Scientific Committee for Food Safety (Vitenskapskomiteen for mattrygghet, VKM) has appointed an ad hoc group consisting of both VKM members and external experts to answer the request from the Norwegian Food Safety Authority. The members of the ad hoc group are acknowledged for their valuable work on this opinion. The members of the ad hoc group are: VKM members Brit Hjeltnes (Chair), Panel on Animal Health and Welfare Ulf Erikson, Panel on Animal Health and Welfare Stein Mortensen, Panel on Animal Health and Welfare External experts Grete Bæverfjord, Nofima Marin, Sunndalsøra Trond Rosten, SINTEF Fisheries and Aquaculture Peter Østergård, Sp/F Aquamed, Faroe Islands Other contributors to the assessment are Frode Mathisen, Anders Fjellheim and Brit Tørud. -

What Are the Health Effects from Exposure to Carbon Monoxide?
CO Lesson 2 CARBON MONOXIDE: LESSON TWO What are the Health Effects from Exposure to Carbon Monoxide? LESSON SUMMARY Carbon monoxide (CO) is an odorless, tasteless, colorless and nonirritating Grade Level: 9 – 12 gas that is impossible to detect by an exposed person. CO is produced by the Subject(s) Addressed: incomplete combustion of carbon-based fuels, including gas, wood, oil and Science, Biology coal. Exposure to CO is the leading cause of fatal poisonings in the United Class Time: 1 Period States and many other countries. When inhaled, CO is readily absorbed from the lungs into the bloodstream, where it binds tightly to hemoglobin in the Inquiry Category: Guided place of oxygen. CORE UNDERSTANDING/OBJECTIVES By the end of this lesson, students will have a basic understanding of the physiological mechanisms underlying CO toxicity. For specific learning and standards addressed, please see pages 30 and 31. MATERIALS INCORPORATION OF TECHNOLOGY Computer and/or projector with video capabilities INDIAN EDUCATION FOR ALL Fires utilizing carbon-based fuels, such as wood, produce carbon monoxide as a dangerous byproduct when the combustion is incomplete. Fire was important for the survival of early Native American tribes. The traditional teepees were well designed with sophisticated airflow patterns, enabling fires to be contained within the shelter while minimizing carbon monoxide exposure. However, fire was used for purposes other than just heat and cooking. According to the historian Henry Lewis, Native Americans used fire to aid in hunting, crop management, insect collection, warfare and many other activities. Today, fire is used to heat rocks used in sweat lodges. -

Hematology Notes Blood/ Hematology Danil Hammoudi.MD
Hematology notes Blood/ Hematology Danil Hammoudi.MD HTTP://Sinoemedicalassociation.or/AP2/ Page | 1 Blood is a connective tissue whose matrix is fluid. It is composed of: 1. red corpuscles, 2. white cells, 3. platelets, 4. blood plasma. It is transported throughout the body within blood vessels. • Blood is sometimes considered to be a fluid connective tissue because of the mesenchymal origin of its cells and a low ratio of cells to liquid intercellular substance, the blood plasma. • In human adults about 5 liter of blood contribute 7-8 % to the body weight of the individual. • The contribution of red blood cells (erythrocytes) to the total volume of the blood (haematocrit) is about 43%. • Erythrocytes are the dominant (99%) but not the only type of cells in the blood. • We also find leukocytes and, in addition, blood platelets. Erythrocytes, leukocytes and blood platelets are also being referred to as the formed elements of the blood. • Erythrocytes and blood platelets perform their functions exclusively in the blood stream. • In contrast, leukocytes reside only temporarily in the blood. • Leukocytes can leave the blood stream through the walls of capillaries and venules and enter either connective or lymphoid tissues. Hematology notes Page | 2 Hematology notes Page | 3 Blood facts • Approximately 8% of an adult's body weight is made up of blood. • Females have around 4-5 litres, while males have around 5-6 litres. This difference is mainly due to the differences in body size between men and women. • Its mean temperature is 38 degrees Celcius. • It has a pH of 7.35-7.45, making it slightly basic (less than 7 is considered acidic). -

Physiologic Basis of DLCO Testing
The Physiologic Basis of DLCO testing Brian Graham Division of Respirology, Critical Care and Sleep Medicine University of Saskatchewan Objectives • Review gas transport from inhaled gas to the rest of the body body • Review the methods of measuring gas exchange • Review the principles of the DLCO test • Review physiologic factors that affect DLCO Gas exchange pathway 1. Transport from the mouth through the airways of the lung to the alveoli by convective and diffusive gas flow and mixing 2. Diffusion across the surfactant layer and the Type 1 pneumocytes which form the alveolar wall 3. Diffusion through the interstitium between the alveolar wall and the capillary wall 4. Diffusion across the pulmonary capillary endothelium 5. Diffusion through the plasma to the red blood cell 6. Diffusion across the red blood cell membrane 7. Diffusion through the red blood cell cytoplasm to the Hb molecule 8. Binding with a Hb molecule 9. Transport via the circulatory system to the rest of the body http://depts.washington.edu/envh/lung.html interstitium surfactant layer capillary endothelium type 1 pneumocyte 100 mmHg oxygen 40 mmHg 40 mmHg carbon dioxide 45 mmHg red blood cell plasma alveolus capillary Fick’s Law of Diffusion diffusive gas flow α Area × Diffusivity × (P1 – P2) / Thickness diffusivity CO2 ~ 20 × O2 P1 P2 The diffusivity of a gas molecule is equal to its solubility divided by the square root of its molecular weight https://www.easyways.net https://www.anatomynote.com https://www.differencebetween.com Hemoglobin transport • 15 gm Hb in 100 mL of blood with a PO2 of 100 mmHg carries 20 mL of oxygen in contrast to 0.3 mL of oxygen dissolved in 100 mL of plasma • The hemoglobin molecule simultaneously carries O2 and CO2, but not at the same binding sites. -

Respiratory Physiology
Physiology Unit 4 RESPIRATORY PHYSIOLOGY Respiraon • External respiraon – ven3laon – gas exchange • Internal respiraon – cellular respiraon – gas exchange • Respiratory Cycle – Inspiraon • Moving atmospheric air into the lungs – Expiraon • Moving air out of the lungs Lungs vs. Balloons • A lung is similar to a balloon in that it resists stretch, tending to collapse almost totally unless held inflated by a pressure difference between its inside and outside • Lungs and the chest have elas3c proper3es Lung Compliance • Compliance – Elas3city – Tendency to recoil – Tendency of an elas3c structure to oppose stretching or distor3on * Resists distension • Surface tension * Resists distension - Surfactant • Reduces surface tension • Increases compliance (makes them easier to stretch) Airway Resistance F = ΔP/R • Same variables that affect resistance in blood vessels – Tube length, tube radius, fric3on – Tube radius most important factor • Airway resistance is so small that small pressure differences produce large volumes of air flow – Average atmosphere-to-alveoli pressure difference is 1 mmHg, but 500 mL of air is moved (%dal volume) – Low pressure and low resistance • Pulmonary 1/10th of systemic vascular resistance Ven3laon • Exchange of air between atmosphere and alveoli • Atmospheric air pressure is 760 mmHg at sea level • Air moves by bulk flow – F = ΔP/R – F = (Palv – Patm)/R Boyle’s Law • Boyle’s law = (P/V) • Pressure of a given quan3ty of gas is inversely propor3onal to volume • An increase in the volume of the container (lungs) decreases the pressure of the gas (air) Ven3laon Mechanics • Lung volume depends on: 1. Transpulmonary pressure (Ptp) • Inside to outside of the lung • Ptp = Palv – Pip • The force that keeps the lungs inflated • Transmural pressure – Across the wall 2. -

Chapter 16 I. the Respiratory System Respiratory System Respiration Gas Exchange in Lungs Alveoli
10/24/11 I. The Respiratory System Chapter 16 Respiratory Physiology Lecture PowerPoint Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Respiratory System Respiration • Includes: • Divided into: – Ventilation (breathing) – Gas exchange between blood and lungs and – Respiratory zone: site of gas exchange between blood and tissues – Oxygen utilization by tissues to make ATP – Conduction zone: gets air to the respiratory • Ventilation and gas exchange in lungs = zone external respiration • Oxygen utilization and gas exchange in tissues = internal respiration Gas Exchange in Lungs Alveoli • Occurs via diffusion • Air sacs in the lungs where gas exchange occurs • O2 concentration is higher in the lungs than in the blood, so O2 diffuses into blood. • 300 million of them – Provide large surface area (760 square feet) to increase diffusion rate • CO2 concentration in the blood is higher than in the lungs, so CO2 diffuses out of blood. 1 10/24/11 Alveoli Alveoli Capillary within alveolar wall Bronchiole and alveoli Alveolar Cells Conducting Zone • Air travels down the nasal cavity Pharynx • Type I: 95−97% total surface area where Larynx gas exchange occurs Trachea Right and left primary bronchi • Type II: secrete pulmonary surfactant and Secondary bronchi reabsorb sodium and water, preventing Tertiary bronchi (more branching) Terminal bronchioles fluid buildup Respiratory zone (respiratory bronchioles Terminal alveolar sacs Respiratory Structures Trachea and Respiratory Bronchi 2 10/24/11 Functions of Conducting Zone Functions of Conducting Zone • Transports air to the lungs • Warms, humidifies, filters, and cleans the air – Mucus traps small particles, and cilia move it away from the lungs. • Voice production in the larynx as air passes over the vocal folds Thoracic Cavity Thoracic Cavity Cross Section • Contains the heart, trachea, esophagus, and thymus within the central mediastinum • The lungs fill the rest of the cavity. -

Oxygen Transport During Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells Or Artificial Oxygen Carriers
International Journal of Molecular Sciences Review Oxygen Transport during Ex Situ Machine Perfusion of Donor Livers Using Red Blood Cells or Artificial Oxygen Carriers Silke B. Bodewes 1,2 , Otto B. van Leeuwen 1,3, Adam M. Thorne 1,3, Bianca Lascaris 1,3, Rinse Ubbink 3, Ton Lisman 2 , Diethard Monbaliu 4,5 , Vincent E. De Meijer 1 , Maarten W. N. Nijsten 6 and Robert J. Porte 1,* 1 Section of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands; [email protected] (S.B.B.); [email protected] (O.B.v.L.); [email protected] (A.M.T.); [email protected] (B.L.); [email protected] (V.E.D.M.) 2 Surgical Research Laboratory, Department of Surgery, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands; [email protected] 3 Organ Preservation & Resuscitation Unit, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands; [email protected] 4 Department of Abdominal Transplantation Surgery and Coordination, University Hospitals Leuven, 3000 Leuven, Belgium; [email protected] 5 Transplantation Research Group, Department of Microbiology, Immunology, and Transplantation, Katholieke Universiteit Leuven, 3000 Leuven, Belgium 6 Department of Critical Care, University of Groningen, University Medical Center Groningen, 9713 GZ Groningen, The Netherlands; [email protected] * Correspondence: [email protected]; Tel./Fax: +31-50-3611745 Abstract: Oxygenated ex situ machine perfusion of donor livers is an alternative for static cold preservation that can be performed at temperatures from 0 ◦C to 37 ◦C. -

Hemoglobin/Myoglobin Robert F.Diegelmann, Ph.D
Hemoglobin/Myoglobin Robert F.Diegelmann, Ph.D. OBJECTIVES 1. Describe the interactions of heme, globins and oxygen. 2. Discuss the mechanism responsible for SickleCell Anemia. 3. Understand the clinical significance of A1C hemoglobin. 4. Describe the basic biochemical mechanisms of O2 delivery & CO2 removal. RECOMMENDED RESOURCES Lehninger, Principles of Biochemistry, 5th edition, Chapter 5 Molecular Cell Biology, 5th edition; Lodish et al., page 67 http://web.indstate.edu/thcme/mwking/hemoglobinmyoglobin.html#hemoglobin Myoglobin (muscle) & Hemoglobin (Red Blood Cells) were the first proteins for which three dimensional structures were determined. Professor Max Perutz and his colleagues at Cambridge University determined Hemoglobin’s three dimensional structure in the late 1950s Therefore Hemoglobin is one of the most studied & best understood proteins. Figure 1. The Evolution of the Globin protein family Figure 2. Structural similarity of the Globin proteins Figure 3 Below is the basic heme group structure. It consists of a complex organic ring structure named Protoporphyrin. NOTE: Heme metabolism will be covered in more detail in another lecture. Protoporphyrin prosthetic group Porphyrin ring Fe binding site Methene bridge Substitution sites Figure 4 Oxygen is not very soluble in aqueous solutions and therefore needs a special molecule to be carried to tissues and cells. The Protoporphyrin ring structure of Heme binds a single iron atom in its ferrous (Fe 2+) . The iron atom has six coordination bonds, four are found bound to the nitrogens in the Porphyrin ring system and two additional sites perpendicular to the Porphyrin. The Cytochromes (a, b & c) are proteins that also consist of porphyrin structures.