RESPIRATION- Notes I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human Physiology an Integrated Approach
Gas Exchange and Transport Gas Exchange in the Lungs and Tissues 18 Lower Alveolar P Decreases Oxygen Uptake O2 Diff usion Problems Cause Hypoxia Gas Solubility Aff ects Diff usion Gas Transport in the Blood Hemoglobin Binds to Oxygen Oxygen Binding Obeys the Law of Mass Action Hemoglobin Transports Most Oxygen to the Tissues P Determines Oxygen-Hb Binding O2 Oxygen Binding Is Expressed As a Percentage Several Factors Aff ect Oxygen-Hb Binding Carbon Dioxide Is Transported in Three Ways Regulation of Ventilation Neurons in the Medulla Control Breathing Carbon Dioxide, Oxygen, and pH Infl uence Ventilation Protective Refl exes Guard the Lungs Higher Brain Centers Aff ect Patterns of Ventilation The successful ascent of Everest without supplementary oxygen is one of the great sagas of the 20th century. — John B. West, Climbing with O’s , NOVA Online (www.pbs.org) Background Basics Exchange epithelia pH and buff ers Law of mass action Cerebrospinal fl uid Simple diff usion Autonomic and somatic motor neurons Structure of the brain stem Red blood cells and Giant liposomes hemoglobin of pulmonary Blood-brain barrier surfactant (40X) From Chapter 18 of Human Physiology: An Integrated Approach, Sixth Edition. Dee Unglaub Silverthorn. Copyright © 2013 by Pearson Education, Inc. All rights reserved. 633 Gas Exchange and Transport he book Into Thin Air by Jon Krakauer chronicles an ill- RUNNING PROBLEM fated trek to the top of Mt. Everest. To reach the summit of Mt. Everest, climbers must pass through the “death zone” T High Altitude located at about 8000 meters (over 26,000 ft ). Of the thousands of people who have attempted the summit, only about 2000 have been In 1981 a group of 20 physiologists, physicians, and successful, and more than 185 have died. -

The Oxyhaemoglobin Dissociation Curve in Critical Illness
Basic sciences review The Oxyhaemoglobin Dissociation Curve in Critical Illness T. J. MORGAN Intensive Care Facility, Division of Anaesthesiology and Intensive Care, Royal Brisbane Hospital, Brisbane, QUEENSLAND ABSTRACT Objective: To review the status of haemoglobin-oxygen affinity in critical illness and investigate the potential to improve gas exchange, tissue oxygenation and outcome by manipulations of the oxyhaemoglobin dissociation curve. Data sources: Articles and published peer-review abstracts. Summary of review: The P50 of a species is determined by natural selection according to animal size, tissue metabolic requirements and ambient oxygen tension. In right to left shunting mathematical modeling indicates that an increased P50 defends capillary oxygenation, the one exception being sustained hypercapnia. Increasing the P50 should also be protective in tissue ischaemia, and this is supported by modeling and experimental evidence. Most studies of critically ill patients have indicated reduced 2,3-DPG concentrations. This is probably due to acidaemia, and the in vivo P50 is likely to be normal despite low 2,3-DPG levels. It may soon be possible to achieve significant P50 elevations without potentially harmful manipulations of acid-base balance or hazardous drug therapy. Conclusions: Despite encouraging theoretical and experimental data, it is not known whether manipulations of the P50 in critical illness can improve gas exchange and tissue oxygenation or improve outcome. The status of the P50 may warrant more routine quantification and consideration along with the traditional determinants of tissue oxygen availability. (Critical Care and Resuscitation 1999; 1: 93-100) Key words: Critical illness, haemoglobin-oxygen affinity, ischaemia, P50, tissue oxygenation, shunt In intensive care practice, manipulations to improve in the tissues. -

A Cephalopod Approach to Rethinking About the Importance of the Bohr and Haldane Effects·
Pacific Science (1982), vol. 36, no. 3 © 1983 by the University of Hawaii Press. All rights reserved A Cephalopod Approach to Rethinking about the Importance of the Bohr and Haldane Effects· G. LYKKEBOE2 and K. JOHANSEN2 ABSTRACT: This study concerns the physiological implications of the Bohr and Haldane effects and the buffer values in the blood from the cephalopods Nautilus pompilius, Octopus macropus, Sepia latimanus, Nototodarus sloani philippinensis, and Sepioteuthis lessoniana. All species studied except one (Nautilus) have Bohr and Haldane coefficients numerically higher than unity, and the two effects were found to be nearly identical in all cases, in accord with the theoretical prediction of Wyman (1964). However, the functional Haldane coefficient was significantly lower than the Haldane coefficient in two cases (Sepia and Sepioteuthis). Buffer values were highest in the two species with the lowest oxygen requirement (Nautilus and Octopus), whereas the three fast swim mers studied (Nototodarus, Sepia, and Sepioteuthis) display comparatively low buffer values. It is concluded that the large Bohr effects seen in four of the five species may have their primary effect on oxygen loading in the gills. THE OXYGEN AFFINITY ofcephalopod blood is Since the Bohr effect is generally acredited pH-sensitive in all reported cases. However, physiological significance in respiratory blood for no other group of animals does the pH gas transport, variations in it that are related sensitivity ofthe O 2 binding, expressed by the to behavior, habitat, or systemic factors Bohr coefficient (Lllog P50/LlpH), show such a should be easily discernible in cephalopods. large variability between species (P50 denotes The present study compares blood respi the oxygen tension at half02 saturation ofthe ratory properties and discusses their possible blood). -

Risk Assessment of Recirculation Systems in Salmonid Hatcheries
Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Risk Assessment of Recirculation Systems in Salmonid Hatcheries Opinion of the Panel on Animal Health and Welfare of the Norwegian Scientific Committee for Food Safety Date: 10.01.12 Doc. no.: 09-808-Final ISBN: 978-82-8259-048-8 VKM Report 2012: 01 1 Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Risk Assessment of Recirculation Systems in Salmonid Hatcheries Brit Hjeltnes (chair of ad hoc group) Grete Bæverfjord Ulf Erikson Stein Mortensen Trond Rosten Peter Østergård 2 Norwegian Scientific Committee for Food Safety (VKM) Doc.no 09/808-Final Contributors Persons working for VKM, either as appointed members of the Committee or as ad hoc experts, do this by virtue of their scientific expertise, not as representatives for their employers. The Civil Services Act instructions on legal competence apply for all work prepared by VKM. Acknowledgements The Norwegian Scientific Committee for Food Safety (Vitenskapskomiteen for mattrygghet, VKM) has appointed an ad hoc group consisting of both VKM members and external experts to answer the request from the Norwegian Food Safety Authority. The members of the ad hoc group are acknowledged for their valuable work on this opinion. The members of the ad hoc group are: VKM members Brit Hjeltnes (Chair), Panel on Animal Health and Welfare Ulf Erikson, Panel on Animal Health and Welfare Stein Mortensen, Panel on Animal Health and Welfare External experts Grete Bæverfjord, Nofima Marin, Sunndalsøra Trond Rosten, SINTEF Fisheries and Aquaculture Peter Østergård, Sp/F Aquamed, Faroe Islands Other contributors to the assessment are Frode Mathisen, Anders Fjellheim and Brit Tørud. -

Would the Hemoglobin–Oxygen Dissociation Curve Shift with Fetal Hemoglobin Compared with Adult Hemoglobin?
❖ CASE 19 A 23-year-old man with no medical problems is brought to the emergency cen- ter by family members who found him to be confused, nauseated, short of breath, and complaining of a headache. The patient was found in the basement of their home next to a furnace, where he was trying to stay warm on a cold winter day. On examination, the patient is lethargic and confused. His lips appear a bright pink. A urine drug screen is obtained and is negative. His serum carboxyhemoglobin level is elevated. The patient is diagnosed with car- bon monoxide poisoning and is admitted to the hospital for further treatment. ◆ What is the mechanism by which carbon monoxide causes hypoxia? ◆ In which direction (right or left) would the hemoglobin–oxygen dissociation curve shift with fetal hemoglobin compared with adult hemoglobin? ◆ What is the most common way in which carbon dioxide is transported in venous blood? 154 CASE FILES: PHYSIOLOGY ANSWERS TO CASE 19: OXYGEN-CARBON DIOXIDE TRANSPORT Summary: A 23-year-old man has confusion, nausea, shortness of breath, and headache after being found near a furnace in the basement. The patient has clinical and laboratory findings consistent with carbon monoxide poisoning. ◆ Hypoxia with carbon monoxide: Decreased oxygen-binding capacity of hemoglobin. ◆ Fetal hemoglobin–oxygen dissociation curve: Shift to the left. ◆ Most common way carbon dioxide is transported in the blood: - Bicarbonate (HCO3 ). CLINICAL CORRELATION Carbon monoxide is a gas that is produced commonly by internal combustion engines, fossil-fuel home appliances (heaters, stoves, furnaces), and incom- plete combustion of nearly all natural and synthetic products. -

Pranayama Redefined/ Breathing Less to Live More
Pranayama Redefined: Breathing Less to Live More by Robin Rothenberg, C-IAYT Illustrations by Roy DeLeon ©Essential Yoga Therapy - 2017 ©Essential Yoga Therapy - 2017 REMEMBERING OUR ROOTS “When Prana moves, chitta moves. When prana is without movement, chitta is without movement. By this steadiness of prana, the yogi attains steadiness and should thus restrain the vayu (air).” Hatha Yoga Pradipika Swami Muktabodhananda Chapter 2, Verse 2, pg. 150 “As long as the vayu (air and prana) remains in the body, that is called life. Death is when it leaves the body. Therefore, retain vayu.” Hatha Yoga Pradipika Swami Muktabodhananda Chapter 2, Verse 3, pg. 153 ©Essential Yoga Therapy - 2017 “Pranayama is usually considered to be the practice of controlled inhalation and exhalation combined with retention. However, technically speaking, it is only retention. Inhalation/exhalation are methods of inducing retention. Retention is most important because is allows a longer period for assimilation of prana, just as it allows more time for the exchange of gases in the cells, i.e. oxygen and carbon dioxide.” Hatha Yoga Pradipika Swami Muktabodhananda Chapter 2, Verse 2, pg. 151 ©Essential Yoga Therapy - 2017 Yoga Breathing in the Modern Era • Focus tends to be on lengthening the inhale/exhale • Exhale to induce relaxation (PSNS activation) • Inhale to increase energy (SNS) • Big ujjayi - audible • Nose breathing is emphasized at least with inhale. Some traditions teach mouth breathing on exhale. • Focus on muscular action of chest, ribs, diaphragm, intercostals and abdominal muscles all used actively on inhale and exhale to create the ‘yoga breath.’ • Retention after inhale and exhale used cautiously and to amplify the effect of inhale and exhale • Emphasis with pranayama is on slowing the rate however no discussion on lowering volume. -
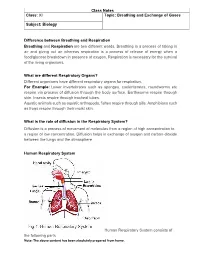
Class Notes Class: XI Topic: Breathing and Exchange of Gases Subject: Biology Difference Between Breathing and Respiration Breat
Class Notes Class: XI Topic: Breathing and Exchange of Gases Subject: Biology Difference between Breathing and Respiration Breathing and Respiration are two different words. Breathing is a process of taking in air and giving out air whereas respiration is a process of release of energy when a food/glucose breakdown in presence of oxygen. Respiration is necessary for the survival of the living organisms. What are different Respiratory Organs? Different organisms have different respiratory organs for respiration. For Example: Lower invertebrates such as sponges, coelenterates, roundworms etc respire via process of diffusion through the body surface. Earthworms respire through skin. Insects respire through tracheal tubes. Aquatic animals such as aquatic arthropods, fishes respire through gills. Amphibians such as frogs respire through their moist skin. What is the role of diffusion in the Respiratory System? Diffusion is a process of movement of molecules from a region of high concentration to a region of low concentration. Diffusion helps in exchange of oxygen and carbon-dioxide between the lungs and the atmosphere. Human Respiratory System Human Respiratory System consists of the following parts Note: The above content has been absolutely prepared from home. • Nostrils is the first part of the respiratory system from where the air enters. • Trachea also known as wind pipe filters the air that is inhaled. Trachea is covered by a lid known as glottis at the time of swallowing of food. It prevents the food from entering into the trachea. It branches into bronchi. • Bronchi also known as air tubes pass air into the lungs. Bronchi branches into bronchioles. -

Respiratory Physiology
Physiology Unit 4 RESPIRATORY PHYSIOLOGY Respiraon • External respiraon – ven3laon – gas exchange • Internal respiraon – cellular respiraon – gas exchange • Respiratory Cycle – Inspiraon • Moving atmospheric air into the lungs – Expiraon • Moving air out of the lungs Lungs vs. Balloons • A lung is similar to a balloon in that it resists stretch, tending to collapse almost totally unless held inflated by a pressure difference between its inside and outside • Lungs and the chest have elas3c proper3es Lung Compliance • Compliance – Elas3city – Tendency to recoil – Tendency of an elas3c structure to oppose stretching or distor3on * Resists distension • Surface tension * Resists distension - Surfactant • Reduces surface tension • Increases compliance (makes them easier to stretch) Airway Resistance F = ΔP/R • Same variables that affect resistance in blood vessels – Tube length, tube radius, fric3on – Tube radius most important factor • Airway resistance is so small that small pressure differences produce large volumes of air flow – Average atmosphere-to-alveoli pressure difference is 1 mmHg, but 500 mL of air is moved (%dal volume) – Low pressure and low resistance • Pulmonary 1/10th of systemic vascular resistance Ven3laon • Exchange of air between atmosphere and alveoli • Atmospheric air pressure is 760 mmHg at sea level • Air moves by bulk flow – F = ΔP/R – F = (Palv – Patm)/R Boyle’s Law • Boyle’s law = (P/V) • Pressure of a given quan3ty of gas is inversely propor3onal to volume • An increase in the volume of the container (lungs) decreases the pressure of the gas (air) Ven3laon Mechanics • Lung volume depends on: 1. Transpulmonary pressure (Ptp) • Inside to outside of the lung • Ptp = Palv – Pip • The force that keeps the lungs inflated • Transmural pressure – Across the wall 2. -

Breathing Issues: in Depth Tutorial Hypo=Low Hyper= High Or More
Breathing Issues: In Depth Tutorial Hypo=low Hyper= high or more In the normal state, when we breathe in, we take in oxygen, the oxygen goes to the lungs and gets absorbed into the circulation, in exchange for carbon dioxide. Then, we exhale the carbon dioxide. One way to think of this is that you take in oxygen and you breathe out carbon dioxide. There are two issues that can occur with breathing problems. One is a low oxygen problem (hyoxemia). The other is retention of carbon dioxide (hypercarbia). A low oxygen and a high carbon dioxide level (in lungs and blood) can sometimes be related. Pulmonologist talk about these two issues using the following terms: 1. you can have a problem with oxygenation AND/OR 2. you can have a problem with ventilation (ventilation means the process by which your lungs expand and by taking in the air you exchange the oxygen for carbon dioxide). Ventilation is the problem for people with neuromuscular disease. Ventilation problems lead to carbon dioxide build up. Ventilation requires you to have the muscle support to expand your lungs during inhalation (your chest wall muscles assist in this effort as does the diaphragm. Scoliosis can impair chest expansion). Depending on how weak you are, you may or not be able to take an effective large enough breath. Because you are not taking a deep enough breath in, when you exhale you have a smaller amount of air to breathe out and it is not enough volume to get rid of the carbon dioxide. The carbon dioxide slowly starts to build up over time. -

Chapter 16 I. the Respiratory System Respiratory System Respiration Gas Exchange in Lungs Alveoli
10/24/11 I. The Respiratory System Chapter 16 Respiratory Physiology Lecture PowerPoint Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Respiratory System Respiration • Includes: • Divided into: – Ventilation (breathing) – Gas exchange between blood and lungs and – Respiratory zone: site of gas exchange between blood and tissues – Oxygen utilization by tissues to make ATP – Conduction zone: gets air to the respiratory • Ventilation and gas exchange in lungs = zone external respiration • Oxygen utilization and gas exchange in tissues = internal respiration Gas Exchange in Lungs Alveoli • Occurs via diffusion • Air sacs in the lungs where gas exchange occurs • O2 concentration is higher in the lungs than in the blood, so O2 diffuses into blood. • 300 million of them – Provide large surface area (760 square feet) to increase diffusion rate • CO2 concentration in the blood is higher than in the lungs, so CO2 diffuses out of blood. 1 10/24/11 Alveoli Alveoli Capillary within alveolar wall Bronchiole and alveoli Alveolar Cells Conducting Zone • Air travels down the nasal cavity Pharynx • Type I: 95−97% total surface area where Larynx gas exchange occurs Trachea Right and left primary bronchi • Type II: secrete pulmonary surfactant and Secondary bronchi reabsorb sodium and water, preventing Tertiary bronchi (more branching) Terminal bronchioles fluid buildup Respiratory zone (respiratory bronchioles Terminal alveolar sacs Respiratory Structures Trachea and Respiratory Bronchi 2 10/24/11 Functions of Conducting Zone Functions of Conducting Zone • Transports air to the lungs • Warms, humidifies, filters, and cleans the air – Mucus traps small particles, and cilia move it away from the lungs. • Voice production in the larynx as air passes over the vocal folds Thoracic Cavity Thoracic Cavity Cross Section • Contains the heart, trachea, esophagus, and thymus within the central mediastinum • The lungs fill the rest of the cavity. -

Respiratory System True Or False
Anatomy & Physiology Test November 4, 2017 Respiratory System True or False: 1. The major factor that stimulates the medulla oblongata and pons to produce respiration is not oxygen concentration, but rather the concentration of carbon dioxide in the blood. 2. The functional residual capacity (FRC) is the amount of air that remains in the lung after a normal tidal expiration; it is the sum of expiratory reserve volume and residual volume. 3. Compared to an atmospheric air, the alveolar air contains less of water vapor but greater amount of carbon dioxide. 4. Binding of the first oxygen molecule causes a conformational change in hemoglobin that allows the second molecule of oxygen to bind more readily, however at the tissue level, as the first oxygen molecule dissociates, it is harder for the next oxygen molecule to dissociate. 5. A large fraction—about 70 percent—of the carbon dioxide molecules that diffuse into the blood is transported to the lungs as dissolved state in plasma. 6. It is more difficult for a body to achieve the same level of oxygen saturation at high altitude than at low altitude, due to lower portion of oxygen (5%). Short Answers: 7. Describe how the rise of concentration of carbon dioxide or hydrogen ions affects the respiration centers and the rate and depth of respiration. 8. Explain what happens to perfusion when ventilation to an alveolus is not sufficient. 9. What are the factors that may cause the oxygen–hemoglobin dissociation curve shift to the “RIGHT”? 10. Please complete the following chemical equation: _____ ______ + - Enzyme:______ 11. -

Improved Oxygen Release: an Adaptation of Mature Red Cells to Hypoxia
Improved oxygen release: an adaptation of mature red cells to hypoxia Miles J. Edwards, … , Carrie-Lou Walters, James Metcalfe J Clin Invest. 1968;47(8):1851-1857. https://doi.org/10.1172/JCI105875. Research Article Blood from patients with erythrocytosis secondary to arterial hypoxemia due either to congenital heart disease or to chronic obstructive pulmonary disease was shown to have a decreased affinity for oxygen; the average oxygen pressure required to produce 50% saturation of hemoglobin with oxygen was 29.8 mm Hg (average normal, 26.3 mm Hg). Such a displacement of the blood oxygen equilibrium curve promotes the release of oxygen from blood to the tissues. Studies were also performed upon blood from a man with complete erythrocyte aplasia who received all of his red cells by transfusion from presumably normal persons. With mild anemia (hematocrit, 28%), the affinity of his blood for oxygen was slightly diminished (an oxygen pressure of 27.0 mm Hg was required to produce 50% saturation of hemoglobin with oxygen). With severe anemia (hematocrit, 13.5%), however, his blood had a markedly decreased oxygen affinity (an oxygen pressure of 29.6 mm Hg was required to produce 50% saturation of hemoglobin with oxygen). We conclude that patients with various conditions characterized by an impairment in the oxygen supply system to tissues respond with a diminished affinity of their blood for oxygen. Although the mechanism which brings about this adaptation is not known, the displacement of the oxygen equilibrium curve is associated with an increase in heme-heme interaction. The decrease in blood oxygen affinity […] Find the latest version: https://jci.me/105875/pdf Improved Oxygen Release: an Adaptation of Mature Red Cells to Hypoxia MUas J.