71794 Federal Register / Vol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Library of Congress Classification
R MEDICINE (GENERAL) R Medicine (General) Periodicals. Societies. Serials 5 International periodicals and serials 10 Medical societies Including aims, scope, utility, etc. International societies 10.5.A3 General works 10.5.A5-Z Individual societies America English United States. Canada 11 Periodicals. Serials 15 Societies British West Indies. Belize. Guyana 18 Periodicals. Serials 20 Societies Spanish and Portuguese Latin America 21 Periodicals. Serials 25 Societies 27.A-Z Other, A-Z 27.F7 French Europe English 31 Periodicals. Serials 35 Societies Dutch 37 Periodicals. Serials 39 Societies French 41 Periodicals. Serials 45 Societies German 51 Periodicals. Serials 55 Societies Italian 61 Periodicals. Serials 65 Societies Spanish and Portuguese 71 Periodicals. Serials 75 Societies Scandinavian 81 Periodicals. Serials 85 Societies Slavic 91 Periodicals. Serials 95 Societies 96.A-Z Other European languages, A-Z 96.H8 Hungarian Asia 97 English 97.5.A-Z Other European languages, A-Z 97.7.A-Z Other languages, A-Z Africa 98 English 98.5.A-Z Other European languages, A-Z 98.7.A-Z Other languages, A-Z 1 R MEDICINE (GENERAL) R Periodicals. Societies. Serials -- Continued Australasia and Pacific islands 99 English 99.5.A-Z Other European languages, A-Z 99.7.A-Z Other languages, A-Z Indexes see Z6658+ (101) Yearbooks see R5+ 104 Calendars. Almanacs Cf. AY81.M4 American popular medical almanacs 106 Congresses 108 Medical laboratories, institutes, etc. Class here papers and proceedings For works about these organizations see R860+ Collected works (nonserial) Cf. R126+ Ancient Greek and Latin works 111 Several authors 114 Individual authors Communication in medicine Cf. -

Reframing Sport Contexts: Labeling, Identities, and Social Justice
Reframing Sport Contexts: Labeling, Identities, and Social Justice Dr. Ted Fay and Eli Wolff Sport in Society Disability in Sport Initiative Northeastern University Critical Context • Marginalization (Current Status Quo) vs. • Legitimatization (New Inclusive Paradigm) Critical Context Naturalism vs. Trans-Humanism (Wolbring, G. (2009) How Do We Handle Our Differences related to Labeling Language and Cultural Identities? • Stereotyping? • Prejudice? • Discrimination? (Carr-Ruffino, 2003, p. 1) Ten Major Cultural Differences 1) Source of Control 2) Collectivism or Individualism 3) Homogeneous or Heterogeneous 4) Feminine or Masculine 5) Rank Status 6) Risk orientation 7) Time use 8) Space use 9) Communication Style 10) Economic System (Carr – Ruffino, 2003, p.27) Rationale for Inclusion • Divisioning by classification relative to “fair play” and equity principles • Sport model rather than “ism” segregated model (e.g., by race, gender, disability, socio-economic class, sexual orientation, look (body image), sect (religion), age) • Legitimacy • Human rights and equality Social Dynamics of Inequality Reinforce and reproduce Social Institutions Ideology Political (Patriarchy) Economic Educational Perpetuates Religious Prejudice & Are institutionalized by Discrimination Cultural Practices (ISM) Sport Music Art (Sage, 1998) Five Interlinking Conceptual Frameworks • Critical Change Factors Model (CCFM) • Organizational Continuum in Sport Governance (OCSG) • Criteria for Inclusion in Sport Organizations (CISO) • Individual Multiple Identity Sport Classifications Index (IMISCI) • Sport Opportunity Spectrum (SOS) Critical Change Factors Model (CCFM) F1) Change/occurrence of major societal event (s) affecting public opinion toward ID group. F2) Change in laws, government and court action in changing public policies toward ID group. F3) Change in level of influence of high profile ID group role models on public opinion. -

Pocket Filters Made of Non-Woven Synthetic Fibres – Type
PFS 6.2 – X XPFStestregistrierung Pocket filters made of non- woven synthetic fibres Type PFS Prefilters or final filters in ventilation systems Pocket filters for the separation of fine dust Filter classes M5, M6, F7 Performance data tested to EN 779 AIR FILTERS CLASS M5-F9 Eurovent certification for fine dust filters Meets the hygiene requirements of VDI 6022 Non-woven synthetic fibres, welded 6 Eurovent certification Enlarged filter area due to filter pockets Low initial differential pressure and high dust holding capacity Variable number of pockets and pocket depth H Quick installation and filter changing times due to easy, safe handling ISC GE N TE IE S T Fitting into standard cell frames for filter walls (type SIF) or into universal G E Y T H casings (type UCA) for duct installation Optional equipment and accessories V 2 DI 602 Front frame made of plastic or galvanised sheet steel Tested to VDI 6022 09/2013 – DE/en K7 – 6.2 – 1 Pocket filters made of non-woven synthetic fibres General information PFS Type Page PFS General information 6.2 – 2 Order code 6.2 – 3 Dimensions and weight 6.2 – 4 Specification text 6.2 – 5 Basic information and nomenclature 10.1 – 1 Description Application Materials and surfaces – Pocket filter made of non-woven synthetic – Filter media made of non-woven synthetic fibres type PFS for the separation of fine dust fibres – Fine dust filter: Prefilter or final filter in – Frame made of plastic or galvanised sheet ventilation systems steel Classification Standards and guidelines – Eurovent certification for -

The ICD-10 Classification of Mental and Behavioural Disorders Diagnostic Criteria for Research
The ICD-10 Classification of Mental and Behavioural Disorders Diagnostic criteria for research World Health Organization Geneva The World Health Organization is a specialized agency of the United Nations with primary responsibility for international health matters and public health. Through this organization, which was created in 1948, the health professions of some 180 countries exchange their knowledge and experience with the aim of making possible the attainment by all citizens of the world by the year 2000 of a level of health that will permit them to lead a socially and economically productive life. By means of direct technical cooperation with its Member States, and by stimulating such cooperation among them, WHO promotes the development of comprehensive health services, the prevention and control of diseases, the improvement of environmental conditions, the development of human resources for health, the coordination and development of biomedical and health services research, and the planning and implementation of health programmes. These broad fields of endeavour encompass a wide variety of activities, such as developing systems of primary health care that reach the whole population of Member countries; promoting the health of mothers and children; combating malnutrition; controlling malaria and other communicable diseases including tuberculosis and leprosy; coordinating the global strategy for the prevention and control of AIDS; having achieved the eradication of smallpox, promoting mass immunization against a number of other -

Driving Factors Behind the Purchase of Paint Products : a Case of Indian Paint Shop Presented By: Bala Yogesh S Academic Mentor: Dr
Driving Factors behind the Purchase of Paint Products : A Case of Indian Paint Shop Presented by: Bala Yogesh S Academic Mentor: Dr. Suresh M OBJECTIVE Identified Factors For Paint Buying Behavior Initial Reachability matrix . The customers who visit the dealer shops are • Product Variance (F1) F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 predominantly painters and a few direct consumers. • Service Quality (F2) F1 1 0 0 0 1 1 1 1 0 0 . This paper primarily focus upon identifying the key • Perceived Quality (F3) F2 0 1 0 0 1 0 0 0 1 1 factors that will influence paint buying behavior in a • Perceived Value(F4) F3 0 0 1 1 1 0 0 0 0 0 dealer shop. • Brand Image(F5) F4 0 1 0 1 1 0 0 0 0 0 . In this paper a conceptual model has been developed • Compatible Price (F6) F5 0 0 1 0 1 0 1 0 0 0 using interpretative structural modelling(ISM) • Store Environment (F7) F6 0 0 1 1 0 1 0 0 0 1 approach. This study results indicates product variance • Product Reliability (F8) F7 0 1 0 0 0 0 1 0 1 0 is the key influencing factor, followed by compatible • Customer Value (F9) F8 0 0 1 1 1 0 0 1 0 1 price and product reliability for the purchase decision • Customer Loyalty (F10) F9 0 0 0 0 0 0 0 0 1 1 of paint products by customer. F10 0 0 0 0 0 0 0 0 0 1 Paint Industry WORKING MODEL Final Reachability Matrix Drivin F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 g Power F1 1 1 1 1 1 1 1 1 1 1 10 Identification of Factors F2 0 1 1 1 1 0 1 0 1 1 7 from literature review F3 0 1 1 1 1 0 1 0 1 1 7 F4 0 1 1 1 1 0 1 0 1 1 7 F5 0 1 1 1 1 0 1 0 1 1 7 F6 0 1 1 1 1 1 1 0 1 1 8 F7 0 1 1 1 1 0 1 0 1 1 7 Application of ISM F8 0 1 1 1 1 0 1 1 1 1 8 approach F9 0 0 0 0 0 0 0 0 1 1 2 F10 0 0 0 0 0 0 0 0 0 1 1 . -

Research Article KINEMATIC ANALYSIS of JAVELIN THROW
©Journal of Sports Science and Medicine (2003) 2, 36-46 http://www.jssm.org Research article KINEMATIC ANALYSIS OF JAVELIN THROW PERFORMED BY WHEELCHAIR ATHLETES OF DIFFERENT FUNCTIONAL CLASSES John W. Chow 1 *, Ann F. Kuenster 2 and Young-tae Lim 3 1Department of Exercise and Sport Sciences, University of Florida, Gainesville, USA 2Department of Kinesiology, University of Illinois at Urbana-Champaign, USA 3Division of Sport Science, Konkuk University, Chungju, Korea Received: 10 December 2002 / Accepted: 07 February 2003 / Published (online): 01 June 2003 ABSTRACT The purpose of this study was to identify those kinematic characteristics that are most closely related to the functional classification of a wheelchair athlete and measured distance of a javelin throw. Two S- VHS camcorders (60 field· s-1) were used to record the performance of 15 males of different classes. Each subject performed 6 - 10 throws and the best two legal throws from each subject were selected for analysis. Three-dimensional kinematics of the javelin and upper body segments at the instant of release and during the throw (delivery) were determined. The selection of kinematic parameters that were analyzed in this study was based on a javelin throw model showing the factors that determine the measured distance of a throw. The average of two throws for each subject was used to compute Spearman rank correlation coefficients between selected parameters and measured distance, and between selected parameters and the functional classification. The speeds and angles of the javelin at release, ranged from 9.1 to 14.7 m· s-1 and 29.6 to 35.8º, respectively, were smaller than those exhibited by elite male able-bodied throwers. -
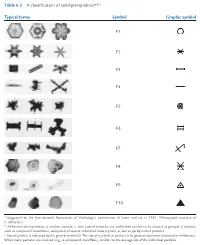
Table 6.2 a Classification of Solid Precipitationa,B,C Typical Forms
P732951-Ch06.qxd 9/12/05 7:44 PM Page 246 246 Cloud Microphysics Table 6.2 A classification of solid precipitationa,b,c Typical forms Symbol Graphic symbol F1 F2 F3 F4 F5 F6 F7 F8 F9 F10 a Suggested by the International Association of Hydrology’s commission of snow and ice in 1951. [Photograph courtesy of V. Schaefer.] b Additional characteristics: p, broken crystals; r, rime-coated particles not sufficiently coated to be classed as graupel; f, clusters, such as compound snowflakes, composed of several individual snow crystals; w, wet or partly melted particles. c Size of particle is indicated by the general symbol D. The size of a crystal or particle is its greatest extension measured in millimeters. When many particles are involved (e.g., a compound snowflake), it refers to the average size of the individual particles. are required. A more efficient technique might be the seeding, but because neither extensive physical to introduce small water droplets (radius Ӎ30 m) nor rigorous statistical evaluations were carried out, or hygroscopic particles (e.g., NaCl) into the base the results were inconclusive. Recently, there has of a cloud; these particles might then grow by been somewhat of a revival of interest in seeding condensation, and then by collision-coalescence, as warm clouds with hygroscopic nuclei to increase they are carried up and subsequently fall through precipitation but, as yet, the efficacy of this tech- a cloud. nique has not been proven. In the second half of the last century, a number of Seeding with hygroscopic particles has been used cloud seeding experiments on warm clouds were in attempts to improve visibility in warm fogs. -

CPI Checklist for Cycles One And
PCGenesis Certified/Classified Personnel Information (CPI) Cycles One and Two Checklist Contact the Technology Management Customer Support Center for assistance as needed. Step Action √ 1 Perform a PCGenesis Data Backup. 2 Verify the CPI Salary Schedule for the current CPI cycle exists. (F4, F5) CPI System Operations Guide, Section D: CPI Salary Schedule Processing 3 Enter, update, and upload the CPI Salary Schedule for the current year, where appropriate. (F4, F5) CPI System Operations Guide, Section D: CPI Salary Schedule Processing 4 Make sure the correct termination dates exist on employees’ payroll and personnel records. Do NOT change the Include on CPI ? flag to N (No) if it is presently Y (Yes). Payroll System Operations Guide, Section B: Payroll Update Processing, Topic 3: Update/Display Personnel Information 5 If the employees’ years should not be incremented: Make sure the Advance/Inhibit flag for the employees’ biographical record is correctly set. (F4, F1) CPI System Operations Guide, Section A: Displaying/Updating Individual Employee Information 6 Print the Certified Employees with Advance/Inhibit Flag of A & E Report. (F4, F7, F9) CPI System Operations Guide, Section E: CPI Report Processing 7 Enter or verify the GaDOE termination date on the CPI Process Control Inquiry screen. (F4, F31, F9) CPI System Operations Guide, Section I: Special Functions 8 Screen-print the CPI Process Control Inquiry screen. (F4, F31, F9) CPI System Operations Guide, Section I: Special Functions 9 Perform the CPI Rollover. (F4, F31, F12) CPI System Operations Guide, Section I: Special Functions 10 Screen-print the CPI Process Control Inquiry screen. (F4, F31, F9) CPI System Operations Guide, Section I: Special Functions 11 OPTIONAL: Update employees’ certificate information using the Professional Standard Commission’s (PSC’s) downloaded file. -

2010 LEGACY/OUTBACK Owner's Manual 2010 LEGACY/OUTBACK Owner's Manual
2010 LEGACY/OUTBACK Owner’s Manual Owner’s 2010 LEGACY/OUTBACK Owner’s Manual A2460BE-A Issued May 2009 Printed in USA 05/09 2010A FUJI HEAVY INDUSTRIES LTD. TOKYO, JAPAN MSA5M1004A 230910_10a_LegacyOutback_OMcover_051109.indd 1 5/12/09 3:13:14 PM Foreword Congratulations on choosing a SUBARU vehicle. This Owner’s Manual has all the information necessary to keep your SUBARU in excellent condition and to properly maintain the emission control system for minimizing emission pollutants. We urge you to read this manual carefully so that you may understand your vehicle and its operation. For information not found in this Owner’s Manual, such as details concerning repairs or adjustments, please contact the SUBARU dealer from whom you purchased your SUBARU or the nearest SUBARU dealer. The information, specifications and illustrations found in this manual are those in effect at the time of printing. FUJI HEAVY INDUSTRIES LTD. reserves the right to change specifications and designs at any time without prior notice and without incurring any obligation to make the same or similar changes on vehicles previously sold. This Owner’s Manual applies to all models and covers all equipment, including factory installed options. Some explanations, therefore may be for equipment not installed in your vehicle. Please leave this manual in the vehicle at the time of resale. The next owner will need the information found herein. FUJI HEAVY INDUSTRIES LTD., TOKYO, JAPAN is a registered trademark of FUJI HEAVY INDUSTRIES LTD. *C Copyright 2009 FUJI HEAVY INDUSTRIES LTD. This manual describes the following types of the Legacy series. -

Library of Congress Classification
G GEOGRAPHY (GENERAL) G Geography (General) For geography and description of individual countries, see D-F For mathematical geography and cartography see GA For physical geography see GB 1 Periodicals. Serials Societies 2 International 3 United States 4 Canada 5 Mexico. Central America. West Indies 6 South America 7 Great Britain 8 Czechoslovakia 9 Austria 10 Hungary 11 France 13 Germany Including West Germany 14 East Germany 15 Greece 17 Italy 19 Belgium 21 Netherlands (Holland) 23 Russia 23.5 Poland 24 Finland 25 Scandinavia 27 Spain 28 Portugal 29 Switzerland 31 Balkan States 32 Turkey 32.2 Jordan 32.5 Israel 32.7 Saudi Arabia 33 China 34 Korea 35 India 36 Pakistan 36.5 Bangladesh 37 Indochina 38 Indonesia 39 Japan 41 South Africa 43 Egypt 45 Algeria 47 Tanzania 49 Other African (not A-Z) 51 Australia 53 New Zealand 55 Pacific islands 56 Congresses Collected works (nonserial) Cf. G159+ Collections of voyages 58 Several authors 59 Individual authors 62 Addresses, essays, lectures 1 G GEOGRAPHY (GENERAL) G 63 Dictionaries. Encyclopedias 64 Directories Geographers 65 Geography as a profession Biography Cf. D-F, History Cf. GA198, GA407, GA473, etc., Cartographers Cf. G200 Explorers 67 Collective 69.A-Z Individual, A-Z e.g. 69.A2 Adamus Bremensis 69.B35 Barkov, Aleksandr Sergeevich 69.B4 Behaim, Martin 69.H2 Hakluyt, Richard 69.K44 Kemmerikh, Aleksandr (Aleksandr Oskarovich), 1912- 69.P4 Petermann, August 69.R4 Rennell, James 69.R6 Ritter, Karl 69.T55 Tillo, Aleksei Andreevich Philosophy. Relation to other topics. Methodology For relation to parapsychology see BF1045.G46 For relation to religion see BL65.G4 For relation to civilization see CB450 For relation to history. -

Women in the 2000, 2004 and 2008 Olympic and Paralympic Games an Analysis of Participation, Leadership and Media Opportunities
September 2009 Women in the 2000, 2004 and 2008 Olympic and Paralympic Games An Analysis of Participation, Leadership and Media Opportunities A Women’s Sports Foundation Research Report Authorship and Acknowledgments TThis report was authored by Maureen Smith, Ph.D., California State University, Sacramento, and Alison M. Wrynn, Ph.D., California State University, Long Beach. The report was reviewed by Donna A. Lopiano, Ph.D.; Don Sabo, Ph.D.; Marjorie A. Snyder, Ph.D.; Linda Mastandrea; Terri Lakowski; Carly Adams, Ph.D., University of Lethbridge; Ellen Carlton, Ph.D., Sonoma State University; Kerrie Kauer, Ph.D., California State University, Long Beach; Cheryl Cooky, Ph.D., California State University, Fullerton; Matthew Llewellyn, Pennsylvania State University; and Jennifer Piatt, Ph.D., California State University, Sacramento. The initial data collection was assisted by graduate students at California State University, Sacramento: Brandon Babcock, Kristi Jouett, Fred Kelley, Louis Lopez, Lindsey McEuen, Rusty Price, K.V. Vigil and Kelli White as well as graduate students from California State University, Long Beach. All data that was obtained from the Internet was accurate as of April 2009. Every attempt was made to obtain the most accurate and up-to-date data for this report. Special thanks to Deana Monahan for her editorial and graphic design expertise. Published September 2009, by the Women’s Sports Foundation® Eisenhower Park, 1899 Hempstead Turnpike, Suite 400 East Meadow, NY 11554 [email protected] www.WomensSportsFoundation.org © 2009, Women’s Sports Foundation, All Rights Reserved This report may be downloaded from www.WomensSportsFoundation.org. This report may be reproduced and distributed only in its entirety. -

An Examination of the Deliberate Practice Framework in Quad Rugby
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 8-2009 An Examination of the Deliberate Practice Framework in Quad Rugby Rachel Lynn Boxell University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Sports Sciences Commons Recommended Citation Boxell, Rachel Lynn, "An Examination of the Deliberate Practice Framework in Quad Rugby. " Master's Thesis, University of Tennessee, 2009. https://trace.tennessee.edu/utk_gradthes/23 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Rachel Lynn Boxell entitled "An Examination of the Deliberate Practice Framework in Quad Rugby." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Science, with a major in Sport Studies. Jeffrey T. Fairbrother, Major Professor We have read this thesis and recommend its acceptance: Gene Hayes, Lars Dzikus Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Rachel Lynn Boxell entitled “An examination of the deliberate practice framework in quad rugby.” I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Master of Science, with a major in Sport Studies.