Photovoltaic Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2015-SVTC-Solar-Scorecard.Pdf
A PROJECT OF THE SILICON VALLEY TOXICS COALITION 2015 SOLAR SCORECARD ‘‘ www.solarscorecard.com ‘‘ SVTC’s Vision The Silicon Valley Toxics Coalition (SVTC) believes that we still have time to ensure that the PV sector is safe The PV industry’s rapid growth makes for the environment, workers, and communities. SVTC it critical that all solar companies envisions a safe and sustainable solar PV industry that: maintain the highest sustainability standards. 1) Takes responsibility for the environmental and health impacts of its products throughout their life- cycles, including adherence to a mandatory policy for ‘‘The Purpose responsible recycling. The Scorecard is a resource for consumers, institutional purchasers, investors, installers, and anyone who wants 2) Implements and monitors equitable environmental to purchase PV modules from responsible product and labor standards throughout product supply chains. stewards. The Scorecard reveals how companies perform on SVTC’s sustainability and social justice benchmarks 3) Pursues innovative approaches to reducing and to ensure that the PV manufacturers protect workers, work towards eliminating toxic chemicals in PV mod- communities, and the environment. The PV industry’s ule manufacturing. continued growth makes it critical to take action now to reduce the use of toxic chemicals, develop responsible For over three decades, SVTC has been a leader in recycling systems, and protect workers throughout glob- encouraging electronics manufacturers to take lifecycle al PV supply chains. Many PV companies want to pro- responsibility for their products. This includes protecting duce truly clean and green energy systems and are taking workers from toxic exposure and preventing hazardous steps to implement more sustainable practices. -

Environmental and Economic Benefits of Building Solar in California Quality Careers — Cleaner Lives
Environmental and Economic Benefits of Building Solar in California Quality Careers — Cleaner Lives DONALD VIAL CENTER ON EMPLOYMENT IN THE GREEN ECONOMY Institute for Research on Labor and Employment University of California, Berkeley November 10, 2014 By Peter Philips, Ph.D. Professor of Economics, University of Utah Visiting Scholar, University of California, Berkeley, Institute for Research on Labor and Employment Peter Philips | Donald Vial Center on Employment in the Green Economy | November 2014 1 2 Environmental and Economic Benefits of Building Solar in California: Quality Careers—Cleaner Lives Environmental and Economic Benefits of Building Solar in California Quality Careers — Cleaner Lives DONALD VIAL CENTER ON EMPLOYMENT IN THE GREEN ECONOMY Institute for Research on Labor and Employment University of California, Berkeley November 10, 2014 By Peter Philips, Ph.D. Professor of Economics, University of Utah Visiting Scholar, University of California, Berkeley, Institute for Research on Labor and Employment Peter Philips | Donald Vial Center on Employment in the Green Economy | November 2014 3 About the Author Peter Philips (B.A. Pomona College, M.A., Ph.D. Stanford University) is a Professor of Economics and former Chair of the Economics Department at the University of Utah. Philips is a leading economic expert on the U.S. construction labor market. He has published widely on the topic and has testified as an expert in the U.S. Court of Federal Claims, served as an expert for the U.S. Justice Department in litigation concerning the Davis-Bacon Act (the federal prevailing wage law), and presented testimony to state legislative committees in Ohio, Indiana, Kansas, Oklahoma, New Mexico, Utah, Kentucky, Connecticut, and California regarding the regulations of construction labor markets. -
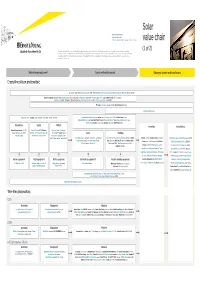
Lsoar Value Chain Value Chain
Solar Private companies in black Public companies in blue Followed by the founding date of companies less than 15 years old value chain (1 of 2) This value chain publication contains information gathered and summarized mainly from Lux Research and a variety of other public sources that we believe to be accurate at the time of ppggyublication. The information is for general guidance only and not intended to be a substitute for detailed research or the exercise of professional judgment. Neither EYGM Limited nor any other member of the global Ernst & Young organization nor Lux Research can accept responsibility for loss to any person relying on this publication. Materials and equipment Components and products Balance of system and installations Crystalline silicon photovoltaic GCL Silicon, China (2006); LDK Solar, China (2005); MEMC, US; Renewable Energy Corporation ASA, Norway; SolarWorld AG, Germany (1998) Bosch Solar Energy, Germany (2000); Canadian Solar, Canada/China (2001); Jinko Solar, China (2006); Kyocera, Japan; Sanyo, Japan; SCHOTT Solar, Germany (2002); Solarfun, China (2004); Tianwei New Energy Holdings Co., China; Trina Solar, China (1997); Yingli Green Energy, China (1998); BP Solar, US; Conergy, Germany (1998); Eging Photovoltaic, China SOLON, Germany (1997) Daqo Group, China; M. Setek, Japan; ReneSola, China (2003); Wacker, Germany Hyundai Heavy Industries,,; Korea; Isofoton,,p Spain ; JA Solar, China (();2005); LG Solar Power,,; Korea; Mitsubishi Electric, Japan; Moser Baer Photo Voltaic, India (2005); Motech, Taiwan; Samsung -

Habitat Connectivity Planning for Selected Focal Species in the Carrizo Plain
Habitat Connectivity Planning for Selected Focal Species in the Carrizo Plain BLM Chuck Graham Chuck Graham Agena Garnett-Ruskovich Advisory Panel Members: Paul Beier, Ph.D., Northern Arizona University Patrick Huber, Ph.D., University of California Davis Steve Kohlmann, Ph.D., Tierra Resource Management Bob Stafford, California Department of Fish and Game Brian Cypher, Ph.D., University of Stanislaus Endangered Species Recovery Program also served as an Advisory Panel Member when this project was under the California Energy Commission’s jurisdiction. Preferred Citation: Penrod, K., W. Spencer, E. Rubin, and C. Paulman. April 2010. Habitat Connectivity Planning for Selected Focal Species in the Carrizo Plain. Prepared for County of San Luis Obsipo by SC Wildlands, http://www.scwildlands.org Habitat Connectivity Planning for Selected Focal Species in the Carrizo Plain Table of Contents 1. Executive Summary 2. Introduction 2.1. Background and Project Need 3. Project Setting 3.1. The Study Area 3.1.1. Location 3.1.2. Physical Features 3.1.3. Biological Features 3.1.4. Human Features 3.2. The Proposed Energy Projects 3.2.1. Topaz Solar Farm 3.2.2. SunPower – California Valley Solar Ranch 4. The Focal Species 4.1. Pronghorn antelope 4.2. Tule elk 4.3. San Joaquin kit fox 5. Conservation Planning Approach 5.1. Modeling Baseline Conditions Of Habitat Suitability And Connectivity For Each Focal Species 5.1.1. Compilation And Refinement Of Digital Data Layers 5.1.2. Modeling Habitat Suitability 5.1.3. Modeling Landscape Permeability 5.1.4. Species-Specific Model Input Data And Conceptual Basis For Model Development 5.1.4.1. -

An Investigation of Solyndra and the Department of Energy Disasters
An Investigation Of Solyndra And The Department Of Energy Disasters By The Internet Revision 1.5 1 A Crime Investigation Table of Contents An Investigation Of Solyndra And The Department Of Energy Disasters................................................1 Overview:...................................................................................................................................................3 Solyndra's Whorehouse Lender..................................................................................................................3 The Solyndra Due Diligence Lie..............................................................................................................15 Goldman Sachs Was The Devil In All Of The Details.............................................................................18 Goldman’s tangled relationship with Tesla draws fire.............................................................................18 The George Mason University Study.......................................................................................................22 Report By The U.S. House of Representatives - Committee on Oversight and Government Reform....32 The Revolving Green Door Payola Scams...............................................................................................69 Google.................................................................................................................................................69 Nancy Ann DeParle.............................................................................................................................69 -

Solar Is Driving a Global Shift in Electricity Markets
SOLAR IS DRIVING A GLOBAL SHIFT IN ELECTRICITY MARKETS Rapid Cost Deflation and Broad Gains in Scale May 2018 Tim Buckley, Director of Energy Finance Studies, Australasia ([email protected]) and Kashish Shah, Research Associate ([email protected]) Table of Contents Executive Summary ......................................................................................................... 2 1. World’s Largest Operational Utility-Scale Solar Projects ........................................... 4 1.1 World’s Largest Utility-Scale Solar Projects Under Construction ............................ 8 1.2 India’s Largest Utility-Scale Solar Projects Under Development .......................... 13 2. World’s Largest Concentrated Solar Power Projects ............................................... 18 3. Floating Solar Projects ................................................................................................ 23 4. Rooftop Solar Projects ................................................................................................ 27 5. Solar PV With Storage ................................................................................................. 31 6. Corporate PPAs .......................................................................................................... 39 7. Top Renewable Energy Utilities ................................................................................. 44 8. Top Solar Module Manufacturers .............................................................................. 49 Conclusion ..................................................................................................................... -

Final Environmental Impact Statement
DOE/EIS–0458 FINAL ENVIRONMENTAL IMPACT STATEMENT VOLUME II: APPENDICES DEPARTMENT OF ENERGY LOAN GUARANTEE TO ROYAL BANK OF SCOTLAND FOR CONSTRUCTION AND STARTUP OF THE TOPAZ SOLAR FARM SAN LUIS OBISPO COUNTY, CALIFORNIA US Department of Energy, Lead Agency Loan Guarantee Program Office Washington, DC 20585 In Cooperation with US Army Corps of Engineers San Francisco District August 2011 APPENDICES TABLE OF CONTENTS Appendix A Public Scoping Appendix B PG&E Connected Action Appendix C Farmlands Correspondence and Analysis Appendix D Visual Simulation Methodology Appendix E Biological Resources, Including Section 7 Consultation Appendix F Cultural Resources, Including Section 106 Consultation Appendix G Draft Wildfire Management Plan Appendix H USACE CWA Section 404 Individual Permit Information Appendix I Contractor Disclosure Statement Appendix J Distribution List Appendix K Mitigation Monitoring and Reporting Plan Appendix A Public Scoping 65306 Federal Register / Vol. 75, No. 204 / Friday, October 22, 2010 / Notices required by Section 10(a)(2) of the discussion of recently released IES DEPARTMENT OF ENERGY Federal Advisory Committee Act and is reports will be held from 2:30 p.m. until intended to notify the public of their 4 p.m. The meeting will close to the Notice of Intent To Prepare an opportunity to attend the open portion public from 4 p.m. to 4:45 p.m. for the Environmental Impact Statement for a of the meeting. The public is being election of Chair and Vice Chair. The Proposed Federal Loan Guarantee To given less than 15 days’ notice due to new officers will have a brief Support Construction of the Topaz the need to accommodate the members’ opportunity to address the membership Solar Farm, San Luis Obispo County, schedules. -

World's Largest Solar Power Plant Unveiled in India Elon Musk's Tesla
World's Largest Solar Power Plant Unveiled in "It's time for the human race to enter India the solar system" Dan Quayle, 44th India has become home to the world's largest solar power plant Vice President of the United States of thanks to a 648-megawatt (MW) facility that has come online in America. the southern part of the country. The previous record holder belonged to the Topaz Solar Farm in California that has a capacity of 550 MW. India has been fast-tracking solar power projects in the past few years thanks to a goalof having 20 gigawatts (GW) of solar power capacity installed by 2022. That goal has spurred some very large solar projects, including a 4 GW plant slated to be built over the next 7 years. Planned solar power projects have grown in size, but each plant takes years to pass through different phases of approval, receive funding and ultimately be built. That's why as we hear about plans for solar power plants with capacities in the thousands of megawatts, the largest ones actually built and running are in the > RESOURCES hundreds of megawatts. None of that should take away from the impressiveness of this new power plant though. The facility, Get Involved with ASES Divisions funded by the Adani Group, is located in Kamuthi, Tamil Nadu Locate an ASES Chapter and covers 10 square kilometers. It consists of 2.5 million Get Up-to-Date Incentives individual solar modules and it's estimated that it will generate enough electricity to power 150,000 homes. -

Concentrating Solar Power Tower: Latest Status 3 Report and Survey of Development Trends
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 17 November 2017 doi:10.20944/preprints201710.0027.v2 1 Review 2 Concentrating Solar Power Tower: Latest Status 3 Report and Survey of Development Trends 4 Albert Boretti 1,*, Stefania Castelletto 2 and Sarim Al-Zubaidy 3 5 1 Department of Mechanical and Aerospace Engineering (MAE), Benjamin M. Statler College of 6 Engineering and Mineral Resources, West Virginia University, Morgantown, WV 26506, United States, 7 [email protected]; [email protected] 8 2 School of Engineering, RMIT University, Bundoora, VIC 3083, Australia; [email protected] 9 3 The University of Trinidad and Tobago, Trinidad and Tobago; [email protected] 10 * Correspondence: [email protected]; [email protected] 11 Abstract: The paper examines design and operating data of current concentrated solar power (CSP) 12 solar tower (ST) plants. The study includes CSP with or without boost by combustion of natural gas 13 (NG), and with or without thermal energy storage (TES). The latest, actual specific costs per 14 installed capacity are very high, 6085 $/kW for Ivanpah Solar Electric Generating System (ISEGS) 15 with no TES, and 9227 $/kW for Crescent Dunes with TES. The actual production of electricity is 16 very low and much less than the expected. The actual capacity factors are 22% for ISEGS, despite 17 combustion of a significant amount of NG largely exceeding the planned values, and 13% for 18 Crescent Dunes. The design values were 33% and 52%. The study then reviews the proposed 19 technology updates to produce better ratio of solar field power to electric power, better capacity 20 factor, better matching of production and demand, lower plant’s cost, improved reliability and 21 increased life span of plant’s components. -

Solar Technology Reference Guide
Solar Technology Reference Guide January 2012 Aaron Binkley Prepared for and Funded by the NAIOP Research Foundation Help ensure that the NAIOP Research Foundation continues to promote industry success. Thank you for your choosing to download this report. Foundation research and analysis gives industry professionals unique insights in to the current business environment and emerging trends that lead to successful development and communities. Traditional sources of revenue cover only a portion of the costs of producing these reports. Additional support, provided by end users of this research through the Foundation’s Sustainer Fund, helps to ensure that the Foundation will have the funds to continue to proactively address the many research project requests it receives each year. Donate to the Sustainers Fund today! Gift Levels Benefactor Gifts of $2,500 and above (Contributions to the NAIOP Research Foundation Amount: Leader Gifts of $1,000-$2,499 are tax deductible to the extent allowed by law.) Donor Gifts of $500-$999 Sustainer Gifts of $250-$499 Please see below for contribution information. Learn how to become involved in the work of the Foundation. Yes, I am interested in ways I can Please call me to discuss support the work of the Foundation. Please send me information about Becoming a Foundation Governor Underwriting a Foundation project, or major initiative Area of interest __________________________ Making an annual gift How to apply for a research grant Contact Information NAME COMPANY TITLE ADDRESS CITY STATE ZIP PHONE E-MAIL Contribution Information *Make checks payable to NAIOP Research Foundation CARD HOLDER NAME CREDIT CARD TYPE NUMBER EXPIRATION DATE Call Bennett Gray at (703) 674-1436 to make a contribution by telephone. -

Solar Photovoltaic Manufacturing: Industry Trends, Global Competition, Federal Support
U.S. Solar Photovoltaic Manufacturing: Industry Trends, Global Competition, Federal Support Michaela D. Platzer Specialist in Industrial Organization and Business January 27, 2015 Congressional Research Service 7-5700 www.crs.gov R42509 U.S. Solar PV Manufacturing: Industry Trends, Global Competition, Federal Support Summary Every President since Richard Nixon has sought to increase U.S. energy supply diversity. Job creation and the development of a domestic renewable energy manufacturing base have joined national security and environmental concerns as reasons for promoting the manufacturing of solar power equipment in the United States. The federal government maintains a variety of tax credits and targeted research and development programs to encourage the solar manufacturing sector, and state-level mandates that utilities obtain specified percentages of their electricity from renewable sources have bolstered demand for large solar projects. The most widely used solar technology involves photovoltaic (PV) solar modules, which draw on semiconducting materials to convert sunlight into electricity. By year-end 2013, the total number of grid-connected PV systems nationwide reached more than 445,000. Domestic demand is met both by imports and by about 75 U.S. manufacturing facilities employing upwards of 30,000 U.S. workers in 2014. Production is clustered in a few states including California, Ohio, Oregon, Texas, and Washington. Domestic PV manufacturers operate in a dynamic, volatile, and highly competitive global market now dominated by Chinese and Taiwanese companies. China alone accounted for nearly 70% of total solar module production in 2013. Some PV manufacturers have expanded their operations beyond China to places like Malaysia, the Philippines, and Mexico. -

Expanding the Photovoltaic Supply Chain in the United States: Opportunities and Challenges
Expanding the Photovoltaic Supply Chain in the United States: Opportunities and Challenges Brittany L. Smith and Robert Margolis NREL is a national laboratory of the U.S. Department of Energy Technical Report Office of Energy Efficiency & Renewable Energy NREL/TP-6A20-73363 Operated by the Alliance for Sustainable Energy, LLC July 2019 This report is available at no cost from the National Renewable Energy Laboratory (NREL) at www.nrel.gov/publications. Contract No. DE-AC36-08GO28308 Expanding the Photovoltaic Supply Chain in the United States: Opportunities and Challenges Brittany L. Smith and Robert Margolis Suggested Citation Smith, Brittany L., and Robert Margolis. (2019). Expanding the Photovoltaic Supply Chain in the United States: Opportunities and Challenges. Golden, CO: National Renewable Energy Laboratory. NREL/TP-6A20-73363. https://www.nrel.gov/docs/fy19osti/73363.pdf. NREL is a national laboratory of the U.S. Department of Energy Technical Report Office of Energy Efficiency & Renewable Energy NREL/TP-6A20-73363 Operated by the Alliance for Sustainable Energy, LLC July 2019 This report is available at no cost from the National Renewable Energy National Renewable Energy Laboratory Laboratory (NREL) at www.nrel.gov/publications. 15013 Denver West Parkway Golden, CO 80401 Contract No. DE-AC36-08GO28308 303-275-3000 • www.nrel.gov NOTICE This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36- 08GO28308. Funding provided by the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Solar Energy Technologies Office.