An Integrated Conservation Approach to Secure Wild Food Plants for Food Security and Nutrition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pandanus Ific Food Leaflet N° Pac 6 ISSN 1018-0966
A publication of the Healthy Pacific Lifestyle Section of the Secretariat of the Pacific Community Pandanus ificifoodileafieiin° Pac i6 ISSN 1018-0966 n parts of the central and northern Pacific, pandanus is a popular food item used in a variety of interesting ways. However, on many other IPacific Islands, pandanus is not well-known as a food. There are many varieties of pandanus, but only In Kiribati, pandanus is called the ‘tree of life’ as it some have edible fruits and nuts. The plants have provides food, shelter and medicine. In the Marshall a distinctive shape and the near-coastal species, Islands, it is called the ‘divine tree’, like coconut, Pandanus tectorius, is found on most Pacific Islands. because of its important role in everyday life. Pandanus The bunches of fruit have many sections called ‘keys’, is also an important staple food in the Federated States which weigh from around 60 to 200 grams each. of Micronesia (FSM), Tuvalu, Tokelau and Papua New (The botanical term for these keys is phalanges, which Guinea. Dried pandanus was once an important food means ‘finger bones’.) People often eat the keys raw, for voyagers on outrigger canoes, enabling seafarers of but the juicy pulp can also be extracted and cooked long ago to survive long journeys. or preserved. The nuts of some varieties are also eaten. In some countries, a number of pandanus varieties are conserved in genebank collections. This leaflet focuses on the Pandanus tectorius species of pandanus. However, other species, such as The pandanus plant plays an important role in Pandanus conoideus and Pandanus jiulianettii, which everyday life in the Pacific. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

422 Part 180—Tolerances and Ex- Emptions for Pesticide
Pt. 180 40 CFR Ch. I (7–1–16 Edition) at any time before the filing of the ini- 180.124 Methyl bromide; tolerances for resi- tial decision. dues. 180.127 Piperonyl butoxide; tolerances for [55 FR 50293, Dec. 5, 1990, as amended at 70 residues. FR 33360, June 8, 2005] 180.128 Pyrethrins; tolerances for residues. 180.129 o-Phenylphenol and its sodium salt; PART 180—TOLERANCES AND EX- tolerances for residues. 180.130 Hydrogen Cyanide; tolerances for EMPTIONS FOR PESTICIDE CHEM- residues. ICAL RESIDUES IN FOOD 180.132 Thiram; tolerances for residues. 180.142 2,4-D; tolerances for residues. Subpart A—Definitions and Interpretative 180.145 Fluorine compounds; tolerances for Regulations residues. 180.151 Ethylene oxide; tolerances for resi- Sec. dues. 180.1 Definitions and interpretations. 180.153 Diazinon; tolerances for residues. 180.3 Tolerances for related pesticide chemi- 180.154 Azinphos-methyl; tolerances for resi- cals. dues. 180.4 Exceptions. 180.155 1-Naphthaleneacetic acid; tolerances 180.5 Zero tolerances. for residues. 180.6 Pesticide tolerances regarding milk, 180.163 Dicofol; tolerances for residues. eggs, meat, and/or poultry; statement of 180.169 Carbaryl; tolerances for residues. policy. 180.172 Dodine; tolerances for residues. 180.175 Maleic hydrazide; tolerances for resi- Subpart B—Procedural Regulations dues. 180.176 Mancozeb; tolerances for residues. 180.7 Petitions proposing tolerances or ex- 180.178 Ethoxyquin; tolerances for residues. emptions for pesticide residues in or on 180.181 Chlorpropham; tolerances for resi- raw agricultural commodities or proc- dues. essed foods. 180.182 Endosulfan; tolerances for residues. 180.8 Withdrawal of petitions without preju- 180.183 Disulfoton; tolerances for residues. -

Introduction
Introduction R. Michael Bourke and Bryant Allen Agriculture is the most important activity carried together in a single publication where it would be out by the vast majority of Papua New Guineans. easy to access. It began as a compendium of statistics For most people, agriculture fills their lives, but has evolved into a comprehensive book of 72 physically, culturally, economically, socially and sections on PNG agriculture and related topics. The nutritionally. Yet agriculture is the most undervalued number of sections alone demonstrates how agricul- and misunderstood part of PNG life (see Twenty ture pervades almost every aspect of life in PNG. myths about PNG agriculture, page 1). The reasons Sections include information about population; for this are partly because mineral and oil exports land use; climate and other aspects of the physical make PNG comparatively wealthy for a developing environment in which agriculture occurs; overviews country; partly because agriculture is practised in of subsistence food production, with detailed the countryside, away from towns, and is therefore descriptions of production techniques and the most largely ‘invisible’ to urban people and international important food crops; descriptions of cash crop visitors; and partly because agriculture is viewed as production for both domestic and export markets; not being ‘modern’. agriculture in the broader economy; and a series of However, as this book shows, agriculture feeds most papers on agricultural development, policies and Papua New Guineans, houses them, provides a governance. An overview of 50 000 years of agricul- significant amount of food to townspeople and earns tural history in PNG follows this introduction. -

Effect of Forced Convection Roasting on Physicochemical and Antioxidant Properties of Whole Grain Maize (Zea Mays L.) and Optimisation of Roasting Conditions
Effect of forced convection roasting on physicochemical and antioxidant properties of whole grain maize (Zea mays L.) and optimisation of roasting conditions by Shuaibu Mallam Bala Dissertation presented for the degree of Doctor of Philosophy (Food Science) in the Faculty of AgriSciences at Stellenbosch University Supervisor: Prof. Marena Manley Co-Supervisor: Prof. Umezuruike Linus Opara March 2016 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Shuaibu Mallam Bala March 2016 Copyright © 2016 Stellenbosch University All rights reserved i Stellenbosch University https://scholar.sun.ac.za Acknowledgements All praises and gratitude are due to Allah, The Almighty. May His peace and blessings be upon Prophet Muhammad (SAW). It has really been a long and tedious journey. AhamdulilLah! I earnestly thank and appreciate the enormous contributions of the following people and organisations towards the success of my academic carrier in particular and life in general: My parents, for taking good care of me from the cradle and continuous affection, prayers, encouragement and constructive advice; my siblings and relatives for support, motivation and encouragement; A special thanks to my beloved wife (Ruqayyah) and daughter (Amirah) for their unconditional love, concern, perseverance, patience, good will, trust, advice and having full confidence in me; well appreciated indeed! My supervisor, Prof. -

Ecological and Ethnobotanical Facet of 'Kelapa Hutan' (Pandanus Spp
OPEN ACCESS International Journal of Botany ISSN 1811-9700 DOI: 10.3923/ijb.2017.103.114 Research Article Ecological and Ethnobotanical Facet of ‘Kelapa Hutan’ (Pandanus Spp.) and Perspectives Towards its Existence and Benefit 1Krisma Lekitoo, 2Hans Fence Zakeus Peday, 2Novita Panambe and 2Reinardus Liborius Cabuy 1Manokwari Environmental and Forest Research Development Institute, 98314 Manokwari, West Papua Province, Indonesia 2Faculty of Forestry, University of Papua, Gunung Salju St., Amban, 98314 Manokwari, West Papua Province, Indonesia Abstract Background and Objective: Pandanus species are spread across the tropical New Guinea Forest and have long been extracted for food. In this study, the ecological and ethnobotanical aspects of Pandanus spp. were investigated. The objectives of the study were to highlight types of edible Pandanus spp. through their taxonomical characteristics, ecological distribution and fruit properties and to describe how traditional communities manage their existence and traditional values by way of ethnobotany and conservation. Methodology: To identify the potency and distribution of the edible Pandanus, continuous strip-sampling was applied to the sampling plots with an intensity of 5%. Temperature and humidity were measured directly under trees. The edible Pandanus specimens were identified by key experts and identification books. The thermogravimetric and Kjeldahl methods were implemented to identify nutrient contents. A semi-structural interview was implemented, which included questions on the management of Pandanus fruit and its social status among communities. Pearson’s correlation analysis was implemented to identify any relationship between temperature, humidity and fruit productivity. This analysis was performed using R statistical program. Results: Two edible Pandanus species were identified based on each characteristic: Pandanus brosimos Merr. -

Albuca Spiralis
Flowering Plants of Africa A magazine containing colour plates with descriptions of flowering plants of Africa and neighbouring islands Edited by G. Germishuizen with assistance of E. du Plessis and G.S. Condy Volume 62 Pretoria 2011 Editorial Board A. Nicholas University of KwaZulu-Natal, Durban, RSA D.A. Snijman South African National Biodiversity Institute, Cape Town, RSA Referees and other co-workers on this volume H.J. Beentje, Royal Botanic Gardens, Kew, UK D. Bridson, Royal Botanic Gardens, Kew, UK P. Burgoyne, South African National Biodiversity Institute, Pretoria, RSA J.E. Burrows, Buffelskloof Nature Reserve & Herbarium, Lydenburg, RSA C.L. Craib, Bryanston, RSA G.D. Duncan, South African National Biodiversity Institute, Cape Town, RSA E. Figueiredo, Department of Plant Science, University of Pretoria, Pretoria, RSA H.F. Glen, South African National Biodiversity Institute, Durban, RSA P. Goldblatt, Missouri Botanical Garden, St Louis, Missouri, USA G. Goodman-Cron, School of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, RSA D.J. Goyder, Royal Botanic Gardens, Kew, UK A. Grobler, South African National Biodiversity Institute, Pretoria, RSA R.R. Klopper, South African National Biodiversity Institute, Pretoria, RSA J. Lavranos, Loulé, Portugal S. Liede-Schumann, Department of Plant Systematics, University of Bayreuth, Bayreuth, Germany J.C. Manning, South African National Biodiversity Institute, Cape Town, RSA A. Nicholas, University of KwaZulu-Natal, Durban, RSA R.B. Nordenstam, Swedish Museum of Natural History, Stockholm, Sweden B.D. Schrire, Royal Botanic Gardens, Kew, UK P. Silveira, University of Aveiro, Aveiro, Portugal H. Steyn, South African National Biodiversity Institute, Pretoria, RSA P. Tilney, University of Johannesburg, Johannesburg, RSA E.J. -
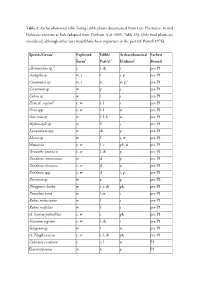
Table 2. Archaeobotanical Table Listing Edible Plants Documented from Late Pleistocene to Mid- Holocene Contexts at Kuk (Adapted from Denham Et Al
Table 2. Archaeobotanical table listing edible plants documented from Late Pleistocene to mid- Holocene contexts at Kuk (adapted from Denham et al. 2003: Table S3). Only food plants are considered, although other uses would have been important in the past (cf. Powell 1976). Species/Genus1 Exploited Edible Archaeobotanical Earliest Form2 Part(s)3 Evidence4 Record Abelmoschus sp. 5 c l, sh s pre-P1 Acalypha sp. w, t l s, p pre-P1 Castanopsis sp. w, t n w, p? pre-P1 Cerastium sp. w ps pre-P1 Coleus sp. w ls pre-P1 Ficus cf. copiosa5 c, w f, l s pre-P1 Ficus spp. c, w l, f w pre-P1 Garcinia sp. w f, l, b w pre-P1 Hydrocotyle sp. w l? s pre-P1 Lycopodium spp. w sh p pre-P1 Maesa sp. w f s, w pre-P1 Musaceae c, w f, c ph, st pre-P1 Oenanthe javanica c, w l, sh p pre-P1 Pandanus antaresensis w dp pre-P1 Pandanus brosimos c, w d p pre-P1 Pandanus spp. c, w d s, p pre-P1 Parsonsia sp. w np pre-P1 Phragmites karka w l, r, sh ph pre-P1 Pouzolzia hirta w l, st s pre-P1 Rubus moluccanus w fs pre-P1 Rubus rosifolius w fs pre-P1 cf. Setaria palmifolia c, w s ph pre-P1 Solanum nigrum c, w l, sh s pre-P1 Syzygium sp. w f w pre-P1 cf. Zingiberaceae c, w r, l, sh ph pre-P1 Colocasia esculenta c c, l st P1 Elaeocarpaceae w np P1 Species/Genus1 Exploited Edible Archaeobotanical Earliest Form2 Part(s)3 Evidence4 Record Ipomoea sp. -

Cheniella Gen. Nov. (Leguminosae: Cercidoideae) from Southern China, Indochina and Malesia
© European Journal of Taxonomy; download unter http://www.europeanjournaloftaxonomy.eu; www.zobodat.at European Journal of Taxonomy 360: 1–37 ISSN 2118-9773 https://doi.org/10.5852/ejt.2017.360 www.europeanjournaloftaxonomy.eu 2017 · Clark R.P. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Research article Cheniella gen. nov. (Leguminosae: Cercidoideae) from southern China, Indochina and Malesia Ruth P. CLARK 1,*, Barbara A. MACKINDER 1,2 & Hannah BANKS 3 1,3 Herbarium, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AE, UK. 2 Royal Botanic Garden, Edinburgh, 20A Inverleith Row, EH3 5LR, UK. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] Abstract. For much of the last thirty years, the caesalpinioid genus Bauhinia has been recognised by numerous authors as a broadly circumscribed, ecologically, morphologically and palynologically diverse pantropical taxon, comprising several subgenera. One of these, Bauhinia subg. Phanera has recently been reinstated at generic rank based on a synthesis of morphological and molecular data. Nevertheless, there remains considerable diversity within Phanera. Following a review of palynological and molecular studies of Phanera in conjunction with a careful re-examination of the morphological heterogeneity within the genus, we have found strong evidence that the species of Phanera subsect. Corymbosae are a natural group that warrant generic status. We describe here the genus Cheniella R.Clark & Mackinder gen. nov. to accommodate them. It comprises 10 species and 3 subspecies, one newly described here. Generic characters include leaves that are simple and emarginate or bilobed; fl owers with elongate hypanthia which are as long as or much longer than the sepals; pods that are glabrous, compressed, oblong, indehiscent or tardily dehiscent; and with numerous seeds, the seeds bearing an unusually long funicle extending most of the way around their circumference. -

Bioactive Components of Pandan's Fruits from Jayawijaya Mountains
IOSR Journal of Environmental Science, Toxicology and Food Technology (IOSR-JESTFT) e-ISSN: 2319-2402,p- ISSN: 2319-2399.Volume 8, Issue 8 Ver. I (Aug. 2014), PP 01-08 www.iosrjournals.org Bioactive Components of Pandan’s Fruits from Jayawijaya Mountains, Papua, Indonesia Been Kogoya1), Bambang Guritno2) Ariffin2) and Agus Suryanto 2) 1(Graduate School of Agriculture, Department of Agriculture, University of Brawijaya, Malang, East Java, Indonesia) 2(Department of Agriculture, University of Brawijaya, Malang, East Java, Indonesia) Abstract: Five Pandan species, namely Pandanus julianettii, Pandanus iwen, Pandanus brosimos, Pandanus sp.1 (owadak) and Pandanus sp.2 (woromo) from Jaya Wijaya Mountains, Papua have potential bioactive components. This study aimed to identify the bioactive components in Pandan’s fleshy receptacle and seed from five Pandan species. Samples were obtained from fleshy receptacle and seed. Sample were mashed and dried for further proximate, minerals, and vitamins contents analysis. The study revealed that nutritive value compositions of food fiber per 100 grams in fleshy receptacle and seed of Pandanus julianettii were 23% and 12%; those of Pandanus sp.1 (owadak) were 17.59% and 18.38%; those of Pandanus sp.2 (woromo) were 30% and 23%; those of Pandanus iwen were 18% and 30%; those of Pandanus brosimos were 47.75 % and 17.40%. The nutritive value compositions of starch per 100 grams in pandan’s fleshy receptacle and seed of Pandanus julianettii were 23 ppm and 0.24 ppm; those of Pandanus sp.1 (owadak) were 26 ppm and 0.96 ppm; those of Pandanus sp.2 (woromo) were 36 ppm and 18 ppm; those of Pandanus iwen were 21 ppm and 0.21 ppm; those of Pandanus brosimos were 35.88 ppm and 9.67 ppm. -

History of Agriculture in Papua New Guinea
History of agriculture in Papua New Guinea R. Michael Bourke sweet potato about 300 years ago; permanent settle- Introduction ment by Europeans and other outsiders, with many introductions of plants and animals after 1870; and the period of rapid social and economic change that The history of agriculture in PNG is about 10 000 commenced about 70 years ago in 1940. years old. This history is reviewed here in the context of 50 000 years of human occupation of the Australia – New Guinea region. 1 More is known about what The peopling of New Guinea has happened nearer to the present, especially since 1870, than about the distant past. Much of the early history (prehistory) of PNG was unknown until When the first humans came to New Guinea about about 50 years ago, but since 1959 there has been 50 000 years ago the climate was very different a lot of research on the prehistory of PNG, with a from now. Worldwide, temperatures were lower, major focus on agriculture. However, this is a rapidly the polar ice caps were larger, glaciers were more evolving field of study and our understanding of common, and sea levels were lower. As a result, the the history of agriculture in PNG is still incomplete. South-East Asia mainland extended as far east as The information that is summarised here will be Bali and Borneo to form a landmass that is known expanded and modified by future research. as Sunda. The Asian mainland (Sunda) and New Historical evidence is reviewed in a number of Guinea were always separated by ocean but, at that periods: the arrival of humans in New Guinea some time, New Guinea was not an island, but formed the 50 000 years ago; the beginnings of agriculture about northern part of a large continent that also included 10 000 years ago; the appearance of Austronesian- Australia and Tasmania, known as Sahul (Figure 1). -

Agriculture in Papua New Guinea: Conditions, Future Prospects and Dispelling Some Myths
INSTITUTE OF NATIONAL AFFAIRS Speech Series No. 19 2009 Agriculture in Papua New Guinea: Conditions, Future Prospects and Dispelling some Myths By Dr Mike Bourke Australian National University July 2009 First published July 2009 Published by: Institute of National Affairs PO Box 1530, Port Moresby NCD Papua New Guinea Copyright © 2009 Institute of National Affairs ISBN 9980-77-162-3 National Library Service – Papua New Guinea Demographic Background • The estimated mid-2008 population for Papua New Guinea was 6.5 million • This consisted of: • 81% rural villagers (5.3 million people) • 13% urban (0.8 million people) • 6% rural non-village (0.4 million people) • Population growth rate is 2.7% pa (1980-2000) Population Altitude Rural Villagers • Based on cash income and other measures, rural villagers may be further sub-divided into: • Not poor - 39% (2.1 million in mid-2008) • Marginally poor 42% (2.2 million in mid-2008) • Extremely poor 18% (1.0 million in mid-2008) • It is people in the first group and some in the second group which produces most of the cash crops and have the highest consumption rate for imported goods. Land use in PNG • In 1975, only 26% of PNG's mass was used for agriculture (fallows, food gardens, tree crops) • This figure has grown to about 30% now • The remaining 70% has never been used for agriculture • This is because it is too swampy, too steep, rainfall and cloud cover are excessive, or it is too high (above 2800 m altitude) • Even within the 30% that can be used for agriculture, there are often severe limitations.