Bioactive Components of Pandan's Fruits from Jayawijaya Mountains
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Front Cover: Pandanus Odoratissimus L.F Published Quarterly PRINTED IN
Front cover: Pandanus odoratissimus L.f (PHOTO: Y.I. ULUMUDDIN) Published quarterly PRINTED IN INDONESIA ISSN: 1412-033X E-ISSN: 2085-4722 BIODIVERSITAS ISSN: 1412-033X Volume 19, Number 1, January 2018 E-ISSN: 2085-4722 Pages: 77-84 DOI: 10.13057/biodiv/d190113 Forest gardens management under traditional ecological knowledge in West Kalimantan, Indonesia BUDI WINARNI1,♥, ABUBAKAR M. LAHJIE2,♥♥, B.D.A.S. SIMARANGKIR2, SYAHRIR YUSUF2, YOSEP RUSLIM2,♥♥♥ 1Department of Agricultural Management, Politeknik Pertanian Negeri Samarinda. Jl. Samratulangi, Kampus Sei Keledang, Samarinda 75131, East Kalimantan, Indonesia. Tel.: +62-541-260421, Fax.: +62-541-260680, ♥email: [email protected] 2Faculty of Forestry, Universitas Mulawarman. Jl. Ki Hajar Dewantara, PO Box 1013, Gunung Kelua, SamarindaUlu, Samarinda 75116, East Kalimantan, Indonesia. Tel.: +62-541-735089, Fax.: +62-541-735379. ♥♥email: [email protected]; ♥♥♥[email protected] Manuscript received: 5 July 2017. Revision accepted: 2 December 2017. Abstract. Winarni B, Lahjie AM, Simarangkir B.D.A.S., Yusuf S, Ruslim Y. 2018. Forest gardens management under traditional ecological knowledge in West Kalimantan, Indonesia. Biodiversitas 19: 77-84. Local wisdom of Dayak Kodatn people in West Kalimantan in forest management shows that human and nature are in one beneficial ecological unity known as Traditional Ecological Knowledge (TEK). Former cultivation forest areas are managed in various ways, including planting forest trees, fruit-producing plants, and rubber trees until they transform -

Nutrient Content of Three Clones of Red Fruit (Pandanus Conoideus) During the Maturity Development
International Food Research Journal 23(3): 1217-1225 (2016) Journal homepage: http://www.ifrj.upm.edu.my Nutrient content of three clones of red fruit (Pandanus conoideus) during the maturity development 1*Sarungallo, Z. L., 1Murtiningrum, 1Santoso, B., 1Roreng, M. K. and 1Latumahina, R. M. M. 1Department of Agricultural Technology, Papua University. Jl. Gunung Salju, Amban, Manokwari-98314, West Papua, Indonesia Article history Abstract Received: 23 November 2014 The purpose of this study was to determine the best harvest time of three clones red fruit Received in revised form: (Pandanus conoideus) based on their nutrient contents. Fruit flesh of three red fruit clones 28 August 2015 (namely Monsor, Edewewits and Memeri) were analyzed the nutritional content, during the Accepted: 9 September 2015 development of the maturity i.e. unripe, half ripe, ripe and overripe. The results show that the maturity stages had a significant effect on the nutrient contents of three clones of red fruit. Nutritional components in the red fruit on are fat (50.8-55.58%), carbohydrate (36.78-46.3%), Keywords vitamin C (24-45 mg per 100 g), phosphorus (654-792 ppm), calcium (4919-5176 ppm), total carotenoids (976-1592 ppm) and total tocopherols (1256-2016 ppm). The changed of nutrient Red fruit (Pandanus conoideus) composition of fruits vary in each clone during ripening. Using the principal component Ripening stages analysis (PCA), commonly three clones of red fruit in unripe and half ripe stages could be Carotenoids characterized by high content of ash, calcium, phosphor, and carbohydrate, while red fruit in Tocopherol maturity level of ripe and overripe were characterized by high content of fat, total carotenoids Nutrient composition and total tocopherol content. -

Pandanus Ific Food Leaflet N° Pac 6 ISSN 1018-0966
A publication of the Healthy Pacific Lifestyle Section of the Secretariat of the Pacific Community Pandanus ificifoodileafieiin° Pac i6 ISSN 1018-0966 n parts of the central and northern Pacific, pandanus is a popular food item used in a variety of interesting ways. However, on many other IPacific Islands, pandanus is not well-known as a food. There are many varieties of pandanus, but only In Kiribati, pandanus is called the ‘tree of life’ as it some have edible fruits and nuts. The plants have provides food, shelter and medicine. In the Marshall a distinctive shape and the near-coastal species, Islands, it is called the ‘divine tree’, like coconut, Pandanus tectorius, is found on most Pacific Islands. because of its important role in everyday life. Pandanus The bunches of fruit have many sections called ‘keys’, is also an important staple food in the Federated States which weigh from around 60 to 200 grams each. of Micronesia (FSM), Tuvalu, Tokelau and Papua New (The botanical term for these keys is phalanges, which Guinea. Dried pandanus was once an important food means ‘finger bones’.) People often eat the keys raw, for voyagers on outrigger canoes, enabling seafarers of but the juicy pulp can also be extracted and cooked long ago to survive long journeys. or preserved. The nuts of some varieties are also eaten. In some countries, a number of pandanus varieties are conserved in genebank collections. This leaflet focuses on the Pandanus tectorius species of pandanus. However, other species, such as The pandanus plant plays an important role in Pandanus conoideus and Pandanus jiulianettii, which everyday life in the Pacific. -

Golden Rules Making the Case for Responsible Mining
GOLDEN RULES Making the case for responsible mining A REPORT BY EARTHWORKS AND OXFAM AMERICA Contents Introduction: The Golden Rules 2 Grasberg Mine, Indonesia 5 Yanacocha Mine, Peru, and Cortez Mine, Nevada 7 BHP Billiton Iron Ore Mines, Australia 9 Hemlo Camp Mines, Canada 10 Mongbwalu Mine, the Democratic Republic of Congo 13 Rosia Montana Mine, Romania 15 Marcopper Mine, the Philippines, and Minahasa Raya and Batu Hijau Mines, Indonesia 17 Porgera Gold Mine, Papua New Guinea 18 Junín Mine, Ecuador 21 Akyem Mine, Ghana 22 Pebble Mine, Alaska 23 Zortman-Landusky Mine, Montana 25 Bogoso/Prestea Mine, Ghana 26 Jerritt Canyon Mine, Nevada 27 Summitville Mine, Colorado 29 Following the rules: An agenda for action 30 Notes 31 Cover: Sadiola Gold Mine, Mali | Brett Eloff/Oxfam America Copyright © EARTHWORKS, Oxfam America, 2007. Reproduction is permitted for educational or noncommercial purposes, provided credit is given to EARTHWORKS and Oxfam America. Around the world, large-scale metals mining takes an enormous toll on the health of the environment and communities. Gold mining, in particular, is one of the dirtiest industries in the world. Massive open-pit mines, some measuring as much as two miles (3.2 kilometers) across, generate staggering quantities of waste—an average of 76 tons for every ounce of gold.1 In the US, metals mining is the leading contributor of toxic emissions to the environment.2 And in countries such as Ghana, Romania, and the Philippines, mining has also been associated with human rights violations, the displacement of people from their homes, and the disruption of traditional livelihoods. -

Building Capacity for Multidisciplinary Landscape Assessment in Papua: Three Phases of Training and Pilot Assessments in the Mamberamo Basin
P.O. Box 6596 JKPWB Tel. (62) 251 622622 Jakarta 10065 Fax (62) 251 622100 CIFOR Indonesia Building Capacity for Multidisciplinary Landscape Assessment in Papua: three phases of training and pilot assessments in the Mamberamo Basin A report based on work jointly undertaken in 2004 by the Center for International Forestry Research, Conservation International (Papua Program), Lembaga Ilmu Pengetahuan Indonesia (LIPI), 18 Papua- based Trainees, and the people of Papasena-I and Kwerba Villages. MLA in Papua, Page 1 SUMMARY Conservation International (CI) supports a number of ongoing initiatives in the Mamberamo area of Papua. The principal aims are to strengthen biodiversity conservation and environmental management and facilitate the creation of a ‘Mamberamo Biodiversity Conservation Corridor’, which links currently established protected areas through strategically placed ‘indigenous forest reserves’. Two primary requirements are 1) to find suitable means to allow local communities to participate in decision-making processes, and 2) capacity building of locally based researchers to assist in planning and developing this program. The MLA training reported here is designed to build capacity and assess options and opportunities within this context. The Centre for International Forestry Research (CIFOR) has developed methods for assessing 'what really matters' to communities living in tropical forest landscapes. Known as the Multidisciplinary Landscape Assessment or ‘MLA' (see http://www.cifor.cgiar.org/mla), this approach enhances understanding amongst conservation and development practitioners, policy makers and forest communities. Information yielded through the MLA can identify where local communities’ interests and priorities might converge (or conflict) with conservation and sustainable development goals. CIFOR's MLA methods have already been applied in Indonesia (East Kalimantan), Bolivia and Cameroon. -

Herbal Medicine: Pandan (Pandanus Tectorius)
For the Month of October Herbal Medicine: Pandan (Pandanus tectorius ) Fragrant Screw Pine The pandan tree grows as tall as 5 meters, with erect, small branches. Pandan is also known as Fragrant Screw Pine. Its trunk bears plenty of prop roots. Its leaves spirals the branches, and crowds at the end. Its male inflorescence emits a fragrant smell, and grows in length for up to 0.5 meters. The fruit of the pandan tree, which is usually about 20 centimeters long, are angular in shape, narrow in the end and the apex is truncate. It grows in the thickets lining the seashores of most places in the Philippines. In various parts of the world, the uses of this plant are very diverse. Some countries concentrate on the culinary uses of pandan, while others deeply rely on its medicinal values. For instance, many Asians regard this food as famine food. Others however mainly associate pandan with the flavoring and nice smell that it secretes. In the Philippines, pandan leaves are being cooked along with rice to in- corporate the flavor and smell to it. As can be observed, the uses of the pandan tree are not limited to cooking uses. Its leaves and roots are found to have medici- nal benefits. Such parts of the plant have been found to have essential oils, tannin, alkaloids and glycosides, which are the reasons for the effective treatment of vari- ous health concerns. It functions as a pain reliever, mostly for headaches and pain caused by arthritis, and even hangover. It can also be used as antiseptic and anti- bacterial, which makes it ideal for healing wounds. -

New Guinea Highland Wild Dogs Are the Original New Guinea Singing Dogs
New Guinea highland wild dogs are the original New Guinea singing dogs Suriani Surbaktia,1, Heidi G. Parkerb,1, James K. McIntyrec, Hendra K. Maurya, Kylie M. Cairnsd, Meagan Selvige, Margaretha Pangau-Adama,e, Apolo Safonpoa, Leonardo Numberia, Dirk Y. P. Runtuboia, Brian W. Davisf,2, and Elaine A. Ostranderb,2 aDepartment of Biology, Universitas Cenderawasih, Jayapura, Papua 99224, Indonesia; bNational Human Genome Research Institute, National Institutes of Health, Bethesda MD 20892; cNew Guinea Highland Wild Dog Foundation, Fernandina Beach, FL 32034; dCentre for Ecosystem Science, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia; eDepartment of Conservation Biology, University of Göttingen, 37073 Göttingen Germany; and fDepartment of Veterinary Integrative Biosciences, Texas A&M University College of Veterinary Medicine, College Station, TX 77843 Contributed by Elaine A. Ostrander, July 17, 2020 (sent for review April 16, 2020; reviewed by Klaus-Peter Koepfli, Fernando Racimo, and Robert D. Schnabel) New Guinea singing dogs (NGSD) are identifiable by their name- on the island. NGSD were first described following collection of sake vocalizations, which are unlike any other canid population. a specimen at an altitude of about 2,100 m in Central Province, Their novel behaviors and potential singular origin during dog PNG, in 1897 (9, 10). Originally classified as a distinct species, domestication make them an attractive, but elusive, subject for Canis hallstromi, their taxonomy remains controversial in part evolutionary and conservation study. Although once plentiful on due to the availability of only captive specimens for genetic the island of New Guinea (NG), they were presumed to currently analysis and debate regarding their origin (3, 10, 11). -

Ecological and Ethnobotanical Facet of 'Kelapa Hutan' (Pandanus Spp
OPEN ACCESS International Journal of Botany ISSN 1811-9700 DOI: 10.3923/ijb.2017.103.114 Research Article Ecological and Ethnobotanical Facet of ‘Kelapa Hutan’ (Pandanus Spp.) and Perspectives Towards its Existence and Benefit 1Krisma Lekitoo, 2Hans Fence Zakeus Peday, 2Novita Panambe and 2Reinardus Liborius Cabuy 1Manokwari Environmental and Forest Research Development Institute, 98314 Manokwari, West Papua Province, Indonesia 2Faculty of Forestry, University of Papua, Gunung Salju St., Amban, 98314 Manokwari, West Papua Province, Indonesia Abstract Background and Objective: Pandanus species are spread across the tropical New Guinea Forest and have long been extracted for food. In this study, the ecological and ethnobotanical aspects of Pandanus spp. were investigated. The objectives of the study were to highlight types of edible Pandanus spp. through their taxonomical characteristics, ecological distribution and fruit properties and to describe how traditional communities manage their existence and traditional values by way of ethnobotany and conservation. Methodology: To identify the potency and distribution of the edible Pandanus, continuous strip-sampling was applied to the sampling plots with an intensity of 5%. Temperature and humidity were measured directly under trees. The edible Pandanus specimens were identified by key experts and identification books. The thermogravimetric and Kjeldahl methods were implemented to identify nutrient contents. A semi-structural interview was implemented, which included questions on the management of Pandanus fruit and its social status among communities. Pearson’s correlation analysis was implemented to identify any relationship between temperature, humidity and fruit productivity. This analysis was performed using R statistical program. Results: Two edible Pandanus species were identified based on each characteristic: Pandanus brosimos Merr. -
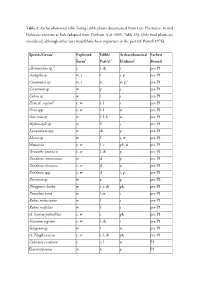
Table 2. Archaeobotanical Table Listing Edible Plants Documented from Late Pleistocene to Mid- Holocene Contexts at Kuk (Adapted from Denham Et Al
Table 2. Archaeobotanical table listing edible plants documented from Late Pleistocene to mid- Holocene contexts at Kuk (adapted from Denham et al. 2003: Table S3). Only food plants are considered, although other uses would have been important in the past (cf. Powell 1976). Species/Genus1 Exploited Edible Archaeobotanical Earliest Form2 Part(s)3 Evidence4 Record Abelmoschus sp. 5 c l, sh s pre-P1 Acalypha sp. w, t l s, p pre-P1 Castanopsis sp. w, t n w, p? pre-P1 Cerastium sp. w ps pre-P1 Coleus sp. w ls pre-P1 Ficus cf. copiosa5 c, w f, l s pre-P1 Ficus spp. c, w l, f w pre-P1 Garcinia sp. w f, l, b w pre-P1 Hydrocotyle sp. w l? s pre-P1 Lycopodium spp. w sh p pre-P1 Maesa sp. w f s, w pre-P1 Musaceae c, w f, c ph, st pre-P1 Oenanthe javanica c, w l, sh p pre-P1 Pandanus antaresensis w dp pre-P1 Pandanus brosimos c, w d p pre-P1 Pandanus spp. c, w d s, p pre-P1 Parsonsia sp. w np pre-P1 Phragmites karka w l, r, sh ph pre-P1 Pouzolzia hirta w l, st s pre-P1 Rubus moluccanus w fs pre-P1 Rubus rosifolius w fs pre-P1 cf. Setaria palmifolia c, w s ph pre-P1 Solanum nigrum c, w l, sh s pre-P1 Syzygium sp. w f w pre-P1 cf. Zingiberaceae c, w r, l, sh ph pre-P1 Colocasia esculenta c c, l st P1 Elaeocarpaceae w np P1 Species/Genus1 Exploited Edible Archaeobotanical Earliest Form2 Part(s)3 Evidence4 Record Ipomoea sp. -

Pacific Colonisation and Canoe Performance: Experiments in the Science of Sailing
PACIFIC COLONISATION AND CANOE PERFORMANCE: EXPERIMENTS IN THE SCIENCE OF SAILING GEOFFREY IRWIN University of Auckland RICHARD G.J. FLAY University of Auckland The voyaging canoe was the primary artefact of Oceanic colonisation, but scarcity of direct evidence has led to uncertainty and debate about canoe sailing performance. In this paper we employ methods of aerodynamic and hydrodynamic analysis of sailing routinely used in naval architecture and yacht design, but rarely applied to questions of prehistory—so far. We discuss the history of Pacific sails and compare the performance of three different kinds of canoe hull representing simple and more developed forms, and we consider the implications for colonisation and later inter-island contact in Remote Oceania. Recent reviews of Lapita chronology suggest the initial settlement of Remote Oceania was not much before 1000 BC (Sheppard et al. 2015), and Tonga was reached not much more than a century later (Burley et al. 2012). After the long pause in West Polynesia the vast area of East Polynesia was settled between AD 900 and AD 1300 (Allen 2014, Dye 2015, Jacomb et al. 2014, Wilmshurst et al. 2011). Clearly canoes were able to transport founder populations to widely-scattered islands. In the case of New Zealand, modern Mäori trace their origins to several named canoes, genetic evidence indicates the founding population was substantial (Penney et al. 2002), and ancient DNA shows diversity of ancestral Mäori origins (Knapp et al. 2012). Debates about Pacific voyaging are perennial. Fifty years ago Andrew Sharp (1957, 1963) was sceptical about the ability of traditional navigators to find their way at sea and, more especially, to find their way back over long distances with sailing directions for others to follow. -

Spring Outfitter 2012 FYI Mechanical - 'Clean', Without Phototags
The original expediTion ouTfiTTer Spring Outfitter 2012 FYI Mechanical - 'clean', without phototags 01/13/12_11:01AM FREE SHIPPING Catalog in-home 2/6/20 12 On yOur purchase Of $75 Or mOre. See inSide for detailS. 2012 OuTfITTEr BOOK: volume 2, no. 1 CD12_1_048A_001.pdf ThE OuTfITTEr BOOK + EXPEDITION TravEl: JOrDaN uPrIsINg + ThE ThIrD DIscIPlINE Of guIDINg sPrINg + KayaK BrazIl: amazONIaN WaTErfalls + JaKE NOrTON TaKEs ON challENgE 21 NO CHANGES CAN BE MADE AT THIS TIME. fEaTurED INTErvIEWs: + caroline george + dave HaHn + melissa arnot + ben stookesberry For changes to the distribution list, please call Mary Ward-Smith at extension 6472. Color is not accurate and is to be used as a guide only. The pages can be viewed by clicking on the page number desired in the Bookmark column. Womens 6 - 19, 24/25, 28/29, 36/37, 42/43, 44, 48 Field & Gear 14/15, 20 - 25, 30/31, 34 - 37, 40 - 43 Mens 6 - 21, 28 - 31, 34/35, 40/41, 45, 48 Kids 46/47 Marketing Components At the end PRSRT STD Prices expire 4/29/12 U.S. POSTAGE PO Box 7001, Groveport, OH 43125 PAID EDDIE BAUER Printed in USA Caring for the environment is one of our core values. The paper in this catalog is certified to contain fiber from responsibly managed sources. Please pass this catalog on or recycle it. THE MICROTHERM™ DOWN VEST NEW CORE FOCUS FROM OUR LIGHTEST, WARMEST LAYER EVER. 800 DOWN FILL MICROTHERM DOWN VEST “THE LIGHTEST, WARMEST THINGS ON EARTH.”® Incredibly thin and lightweight, but with all the insulating warmth of 800 fill Premium European Goose Down in a streamlined, non-puffy silhouette. -

History of Agriculture in Papua New Guinea
History of agriculture in Papua New Guinea R. Michael Bourke sweet potato about 300 years ago; permanent settle- Introduction ment by Europeans and other outsiders, with many introductions of plants and animals after 1870; and the period of rapid social and economic change that The history of agriculture in PNG is about 10 000 commenced about 70 years ago in 1940. years old. This history is reviewed here in the context of 50 000 years of human occupation of the Australia – New Guinea region. 1 More is known about what The peopling of New Guinea has happened nearer to the present, especially since 1870, than about the distant past. Much of the early history (prehistory) of PNG was unknown until When the first humans came to New Guinea about about 50 years ago, but since 1959 there has been 50 000 years ago the climate was very different a lot of research on the prehistory of PNG, with a from now. Worldwide, temperatures were lower, major focus on agriculture. However, this is a rapidly the polar ice caps were larger, glaciers were more evolving field of study and our understanding of common, and sea levels were lower. As a result, the the history of agriculture in PNG is still incomplete. South-East Asia mainland extended as far east as The information that is summarised here will be Bali and Borneo to form a landmass that is known expanded and modified by future research. as Sunda. The Asian mainland (Sunda) and New Historical evidence is reviewed in a number of Guinea were always separated by ocean but, at that periods: the arrival of humans in New Guinea some time, New Guinea was not an island, but formed the 50 000 years ago; the beginnings of agriculture about northern part of a large continent that also included 10 000 years ago; the appearance of Austronesian- Australia and Tasmania, known as Sahul (Figure 1).