Macroevolution of Leaf Defenses and Secondary Metabolites Across the Genus Helianthus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Models Linking Speciation and Extinction to Chromosome and Mating System Evolution
Phylogenetic Models Linking Speciation and Extinction to Chromosome and Mating System Evolution by William Allen Freyman A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology and the Designated Emphasis in Computational and Genomic Biology in the Graduate Division of the University of California, Berkeley Committee in charge: Dr. Bruce G. Baldwin, Chair Dr. John P. Huelsenbeck Dr. Brent D. Mishler Dr. Kipling W. Will Fall 2017 Phylogenetic Models Linking Speciation and Extinction to Chromosome and Mating System Evolution Copyright 2017 by William Allen Freyman Abstract Phylogenetic Models Linking Speciation and Extinction to Chromosome and Mating System Evolution by William Allen Freyman Doctor of Philosophy in Integrative Biology and the Designated Emphasis in Computational and Genomic Biology University of California, Berkeley Dr. Bruce G. Baldwin, Chair Key evolutionary transitions have shaped the tree of life by driving the processes of spe- ciation and extinction. This dissertation aims to advance statistical and computational ap- proaches that model the timing and nature of these transitions over evolutionary trees. These methodological developments in phylogenetic comparative biology enable formal, model- based, statistical examinations of the macroevolutionary consequences of trait evolution. Chapter 1 presents computational tools for data mining the large-scale molecular sequence datasets needed for comparative phylogenetic analyses. I describe a novel metric, the miss- ing sequence decisiveness score (MSDS), which assesses the phylogenetic decisiveness of a matrix given the pattern of missing sequence data. In Chapter 2, I introduce a class of phylogenetic models of chromosome number evolution that accommodate both anagenetic and cladogenetic change. -

Rare and Endemic Plants of Lake County • Serpentine Soil Habitats
OFF_,, C-E COP'-f RARE AND ENDEMIC PLANTS OF LAKE COUNTY • SERPENTINE SOIL HABITATS by Niall F. McCarten Department of Botany • University of California Berkeley, California for • EndangeredPlantProgram California Department of Fish and Game Sacramento, California • RARE AND ENDEMIC PLANTS OF LAKE COUNTY SERPENTINE SOIL HABITATS Prepared by Niall F. McCarten • Departmentof Botany University of California Berkeley, California 94720 • Prepared for Endangered Plant Project California Department of Fish and Game 1416 Ninth Street, Room 1225 • Sacramento, California 95814 Funded by • California Department of Fish and Game Tax Check-off Funds Contract No. C-2037 June 15, 1988 TABLE OF CONTENTS LIST OF TABLES ....................................... ii LIST OF FIGURES ..................................... iii ABSTRACT .......................................... iv INTRODUCTION ...................................... 1 METHODS ......................................... 1 RESULTS ......................................... 2 Rare Plants .................................. 2 Floristics .................................. 5 Plant Communities ............................ 8 Serpentine soils and geology ................... Ii DISCUSSION .......................................... 17 Plant and serpentine soil ecology .............. 17 • Genetics of Serpentine Soil Adaptation ........... 19 Rare Plant Adaptation to Serpentine Soil ......... 19 CONCLUSIONS ............................................. 20 • Causes of Plant Rarity ............................. 21 -

Lntrogression and Its Consequences in Plants Loren H
Botany Publication and Papers Botany 1993 lntrogression and Its Consequences in Plants Loren H. Rieseberg Rancho Santa Ana Botanic Garden Jonathan F. Wendel Iowa State University, [email protected] Follow this and additional works at: http://lib.dr.iastate.edu/bot_pubs Part of the Botany Commons, Evolution Commons, and the Plant Breeding and Genetics Commons Recommended Citation Rieseberg, Loren H. and Wendel, Jonathan F., "lntrogression and Its Consequences in Plants" (1993). Botany Publication and Papers. 8. http://lib.dr.iastate.edu/bot_pubs/8 This Book Chapter is brought to you for free and open access by the Botany at Iowa State University Digital Repository. It has been accepted for inclusion in Botany Publication and Papers by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. lntrogression and Its Consequences in Plants Abstract The or le of introgression in plant evolution has been the subject of considerable discussion since the publication of Anderson's influential monograph, Introgressive Hybridization (Anderson, 1949). Anderson promoted the view, since widely held by botanists, that interspecific transfer of genes is a potent evolutionary force. He suggested that "the raw material for evolution brought about by introgression must greatly exceed the new genes produced directly by mutation" ( 1949, p. 102) and reasoned, as have many subsequent authors, that the resulting increases in genetic diversity and number of genetic combinations promote the development or acquisition of novel adaptations (Anderson, 1949, 1953; Stebbins, 1959; Rattenbury, 1962; Lewontin and Birch, 1966; Raven, 1976; Grant, 1981 ). In contrast to this "adaptationist" perspective, others have accorded little ve olutionary significance to introgression, suggesting instead that it should be considered a primarily local phenomenon with only transient effects, a kind of"evolutionary noise" (Barber and Jackson, 1957; Randolph et al., 1967; Wagner, 1969, 1970; Hardin, 1975). -
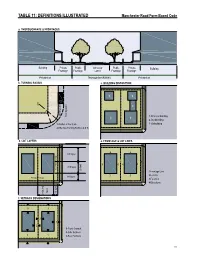
Manchester Road Redevelopment District: Form-Based Code
TaBle 11: deFiniTionS illuSTraTed manchester road Form-Based Code a. ThoroughFare & FronTageS Building Private Public Vehicular Public Private Building Frontage Frontage Lanes Frontage Frontage Private lot Thoroughfare (r.o.w.) Private lot b. Turning radiuS c. Building diSPoSiTion 3 3 2 2 1 Parking Lane Moving Lane 1- Principal Building 1 1 2- Backbuilding 1-Radius at the Curb 3- Outbuilding 2-Effective Turning Radius (± 8 ft) d. loT LAYERS e. FronTage & loT lineS 4 3rd layer 4 2 1 4 4 4 3 2nd layer Secondary Frontage 20 feet 1-Frontage Line 2-Lot Line 1st layer 3 3 Principal Frontage 3-Facades 1 1 4-Elevations layer 1st layer 2nd & 3rd & 2nd f. SeTBaCk deSignaTionS 3 3 2 1 2 1-Front Setback 2-Side Setback 1 1 3-Rear Setback 111 Manchester Road Form-Based Code ARTICLE 9. APPENDIX MATERIALS MBG Kemper Center PlantFinder About PlantFinder List of Gardens Visit Gardens Alphabetical List Common Names Search E-Mail Questions Menu Quick Links Home Page Your Plant Search Results Kemper Blog PlantFinder Please Note: The following plants all meet your search criteria. This list is not necessarily a list of recommended plants to grow, however. Please read about each PF Search Manchesterplant. Some may Road be invasive Form-Based in your area or may Code have undesirable characteristics such as above averageTab insect LEor disease 11: problems. NATIVE PLANT LIST Pests Plants of Merit Missouri Native Plant List provided by the Missouri Botanical Garden PlantFinder http://www.mobot.org/gardeninghelp/plantfinder Master Search Search limited to: Missouri Natives Search Tips Scientific Name Scientific Name Common NameCommon Name Height (ft.) ZoneZone GardeningHelp (ft.) Acer negundo box elder 30-50 2-10 Acer rubrum red maple 40-70 3-9 Acer saccharinum silver maple 50-80 3-9 Titles Acer saccharum sugar maple 40-80 3-8 Acer saccharum subsp. -

Novel Mir390-Dependent Transacting Sirna Precursors in Plants Revealed by a PCR-Based Experimental Approach and Database Analysis
Hindawi Publishing Corporation Journal of Biomedicine and Biotechnology Volume 2009, Article ID 952304, 9 pages doi:10.1155/2009/952304 Research Article Novel miR390-Dependent Transacting siRNA Precursors in Plants Revealed by a PCR-Based Experimental Approach and Database Analysis M. S. Krasnikova,1 I. A. Milyutina,2 V. K. Bobrova, 2 L. V. Ozerova,3 A. V. Troitsky,2 A. G. Solovyev,1, 2 andS.Y.Morozov2 1 Institute of Agricultural Biotechnology, Russian Academy of Agricultural Sciences, Timiryazevskaya 42, 127550 Moscow, Russia 2 A.N. Belozersky Institute of Physico-Chemical Biology, Moscow State University, 119992 Moscow, Russia 3 Main Botanic Garden Russian Academy of Sciences, Botanicheskaya 4, 127276 Moscow, Russia CorrespondenceshouldbeaddressedtoS.Y.Morozov,[email protected] Received 28 February 2009; Accepted 31 July 2009 Recommended by Zhumur Ghosh TAS loci in plant genomes encode transacting small interfering RNAs (ta-siRNAs) that regulate expression of a number of genes. The function of TAS3 precursor in Arabidopsis thaliana is controlled by two miR390 target sites flanking two ta-siARF sequences targeting mRNAs of ARF transcription factors. Cleavage of the 3-miR390-site initiates ta-siRNAs biogenesis. Here we describe the new method for identification of plant ta-siRNA precursors based on PCR with oligodeoxyribonucleotide primers mimicking miR390. The method was found to be efficient for dicotiledonous plants, cycads, and mosses. Based on sequences of amplified loci and a database analysis, a novel type of miR390-dependent TAS sequences was identified in dicots. These TAS loci are characterized by a smaller distance between miR390 sites compared to TAS3, a single copy of ta-siARF, and a sequence conservation pattern pointing to the possibility that processing of novel TAS-like locus is initiated by cleavage of the 5-terminal miR390 target site. -

The Pennsylvania State University
The Pennsylvania State University The Graduate School Intercollege Graduate Degree Program in Plant Biology PHYLOGENOMIC ANALYSIS OF ANCIENT GENOME DUPLICATIONS IN THE HISTORY OF PLANTS A Dissertation in Plant Biology by Yuannian Jiao © 2011 Yuannian Jiao Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy December 2011 The dissertation of Yuannian Jiao was reviewed and approved* by the following: Claude dePamphilis Professor of Biology Dissertation Advisor Chair of Committee Hong Ma Professor of Biology John Carlson Professor of Molecular Genetics Webb Miller Professor of Comparative Genomics and Bioinformatics Naomi Altman Professor of Statistics Teh-hui Kao Chair of Plant Biology Graduate Program *Signatures are on file in the Graduate School iii ABSTRACT Whole-genome duplication (WGD), or polyploidy, followed by gene loss and diploidization, has generally been viewed as a primary source of material for the origin of evolutionary novelties. Most flowering plants have been shown to be ancient polyploids that have undergone one or more rounds of WGDs early in their evolution, and many lineages have since undergone additional, independent and more recent genome duplications. It was suggested that the paleopolyploidy events were crucial to the radiation and success of angiosperms, but evidence for proposed ancient genome duplications remains equivocal. Plant genomes are highly dynamic and usually go through intense structural rearrangements and gene loss following duplication. Old(er) WGDs can not -

Shawnee National Forest Biological Evaluation for Regional Forester's Sensitive Plant Species
SHAWNEE NATIONAL FOREST BIOLOGICAL EVALUATION FOR REGIONAL FORESTER'S SENSITIVE PLANT SPECIES FOREST PLAN REVISION February 3, 2005 The information for the 63 Regional Forester’s sensitive plant species is taken from the Shawnee National Forest list compiled on June 15, 2004, NatureServe (2004), Plants Database (2004), the State heritage database, and available literature. Table 1. Regional Forester’s Sensitive Plant Species (RFSS) on the Forest known to occur or have been documented as historically occurring within the 11 counties of southern Illinois. An asterisk (*) denotes the assumption that the species is extirpated in that county. A double asterisk (**) next to the scientific name indicates that the name follows Mohlenbrock (1986). Note: T = Illinois Threatened, E = Illinois Endangered, EX = presumed extirpated in the State, A = Alexander, G = Gallatin, H = Hardin, Ja = Jackson, Jo = Johnson, M = Massac, Po = Pope, Pu = Pulaski, S = Saline, U = Union, and W = Williamson. Counties IL A G H Ja Jo M Po Pu S U W Scientific Name Common Name E/T X X * X 1. Amorpha nitens Shining false indigo E X X X 2. Asplenium bradleyi Bradley's spleenwort E * * X 3. Asplenium resiliens Black-stem spleenwort E X 4. Bartonia paniculata Twining screwstem E X 5. Berberis canadensis American barberry E X 6. Buchnera americana American bluehearts X 7. Calamagrostis porteri Porter’s reedgrass E ssp. insperata * X * X X 8. Carex communis Fibrous-root sedge T * X X * X 9. Carex decomposita Cypress-knee sedge E X X X X X 10. Carex gigantea Giant sedge E * * * * * * * * * 11. Carex lupuliformis False hop sedge X 12. -

VASCULAR PLANTS of MINNESOTA a Checklist and Atlas
VASCULAR PLANTS of MINNESOTA This page intentionally left blank VASCULAR PLANTS of MINNESOTA A Checklist and Atlas Gerald B. Ownbey and Thomas Morley UNIVERSITY OF MINNESOTA MINNEAPOLIS • LONDON The University of Minnesota Press gratefully acknowledges the generous assistance provided for the publication of this book by the Margaret W. Harmon Fund Minnesota Department of Transportation Minnesota Landscape Arboretum Minnesota State Horticultural Society Olga Lakela Herbarium Fund—University of Minnesota—Duluth Natural Heritage Program of the Minnesota Department of Natural Resources Copyright © 1991 by the Regents of the University of Minnesota. First paperback printing 1992 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the publisher. Published by the University of Minnesota Press 2037 University Avenue Southeast, Minneapolis, MN 55455 Printed in the United States of America on acid-free paper Library of Congress Cataloging-in-Publication Data Ownbey, Gerald B., 1916- Vascular plants of Minnesota : a checklist and atlas / Gerald B. Ownbey and Thomas Morley. p. cm. Includes bibliographical references and index. ISBN 0-8166-1915-8 1. Botany-Minnesota. 2. Phytogeography—Minnesota— Maps. I. Morley, Thomas. 1917- . II. Title. QK168.096 1991 91-2064 582.09776-dc20 CIP The University of Minnesota is an equal-opportunity educator and employer. Contents Introduction vii Part I. Checklist of the Vascular Plants of Minnesota 1 Pteridophytes 3 Gymnosperms 6 Angiosperms 7 Appendix 1. Excluded names 81 Appendix 2. Tables 82 Part II. Atlas of the Vascular Plants of Minnesota 83 Index of Generic and Common Names 295 This page intentionally left blank Introduction The importance of understanding the vegetation of al distributional comments. -

Competitive Effects of Increased Plant Species Richness and Increasd Endemic Versus Native Generalist Species Dominance on the I
COMPETITIVE EFFECTS OF INCREASED PLANT SPECIES RICHNESS AND INCREASD ENDEMIC VERSUS NATIVE GENERALIST SPECIES DOMINANCE ON THE INVASIVE GRASS MICROSTEGIUM VIMINEUM DURING OAK WOODLAND RESTORATION A Thesis Presented in partial fulfillment of requirements for the degree of Master of Science in the Department of Biology The University of Mississippi By: SEAN A. MOYER December 2016 Copyright © 2016 by Sean A. Moyer ALL RIGHTS RESERVED ABSTRACT The hypothesis that species-rich assemblages are resistant to invasion by non-native species has generated considerable research and controversy. However, the relevance of such research to the conservation of biodiversity is questionable, given that local species richness often does not correlate with regional or global species richness, two metrics undoubtedly important to conservation. Furthermore, species of greater conservation interest (i.e. endemics) and widespread generalist species may compete differentially with non-native invasive species. To test whether plant species richness or species fidelity to a regionally rare habitat were more important in competitively suppressing an invasive species, I established a field competition experiment in an oak woodland in north-central Mississippi (USA) between the non-native invasive grass Microstegium vimineum and six native plant species of varying fidelity to fire-maintained open woodlands. Using a split-plot design, dense, established patches of Microstegium were treated with one of the three following native planting treatments or control: (1) a six species polyculture, (2) a monoculture of six individuals of a single species, or (3) a control simulating the soil disturbance of the plantings. I then monitored Microstegium percent cover through the 2015 growing season and into the spring of the following year. -

Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

A Checklist of Vascular Plants Endemic to California
Humboldt State University Digital Commons @ Humboldt State University Botanical Studies Open Educational Resources and Data 3-2020 A Checklist of Vascular Plants Endemic to California James P. Smith Jr Humboldt State University, [email protected] Follow this and additional works at: https://digitalcommons.humboldt.edu/botany_jps Part of the Botany Commons Recommended Citation Smith, James P. Jr, "A Checklist of Vascular Plants Endemic to California" (2020). Botanical Studies. 42. https://digitalcommons.humboldt.edu/botany_jps/42 This Flora of California is brought to you for free and open access by the Open Educational Resources and Data at Digital Commons @ Humboldt State University. It has been accepted for inclusion in Botanical Studies by an authorized administrator of Digital Commons @ Humboldt State University. For more information, please contact [email protected]. A LIST OF THE VASCULAR PLANTS ENDEMIC TO CALIFORNIA Compiled By James P. Smith, Jr. Professor Emeritus of Botany Department of Biological Sciences Humboldt State University Arcata, California 13 February 2020 CONTENTS Willis Jepson (1923-1925) recognized that the assemblage of plants that characterized our flora excludes the desert province of southwest California Introduction. 1 and extends beyond its political boundaries to include An Overview. 2 southwestern Oregon, a small portion of western Endemic Genera . 2 Nevada, and the northern portion of Baja California, Almost Endemic Genera . 3 Mexico. This expanded region became known as the California Floristic Province (CFP). Keep in mind that List of Endemic Plants . 4 not all plants endemic to California lie within the CFP Plants Endemic to a Single County or Island 24 and others that are endemic to the CFP are not County and Channel Island Abbreviations . -

Ecological Checklist of the Missouri Flora for Floristic Quality Assessment
Ladd, D. and J.R. Thomas. 2015. Ecological checklist of the Missouri flora for Floristic Quality Assessment. Phytoneuron 2015-12: 1–274. Published 12 February 2015. ISSN 2153 733X ECOLOGICAL CHECKLIST OF THE MISSOURI FLORA FOR FLORISTIC QUALITY ASSESSMENT DOUGLAS LADD The Nature Conservancy 2800 S. Brentwood Blvd. St. Louis, Missouri 63144 [email protected] JUSTIN R. THOMAS Institute of Botanical Training, LLC 111 County Road 3260 Salem, Missouri 65560 [email protected] ABSTRACT An annotated checklist of the 2,961 vascular taxa comprising the flora of Missouri is presented, with conservatism rankings for Floristic Quality Assessment. The list also provides standardized acronyms for each taxon and information on nativity, physiognomy, and wetness ratings. Annotated comments for selected taxa provide taxonomic, floristic, and ecological information, particularly for taxa not recognized in recent treatments of the Missouri flora. Synonymy crosswalks are provided for three references commonly used in Missouri. A discussion of the concept and application of Floristic Quality Assessment is presented. To accurately reflect ecological and taxonomic relationships, new combinations are validated for two distinct taxa, Dichanthelium ashei and D. werneri , and problems in application of infraspecific taxon names within Quercus shumardii are clarified. CONTENTS Introduction Species conservatism and floristic quality Application of Floristic Quality Assessment Checklist: Rationale and methods Nomenclature and taxonomic concepts Synonymy Acronyms Physiognomy, nativity, and wetness Summary of the Missouri flora Conclusion Annotated comments for checklist taxa Acknowledgements Literature Cited Ecological checklist of the Missouri flora Table 1. C values, physiognomy, and common names Table 2. Synonymy crosswalk Table 3. Wetness ratings and plant families INTRODUCTION This list was developed as part of a revised and expanded system for Floristic Quality Assessment (FQA) in Missouri.