21 CFR Ch. I (4–1–20 Edition)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Non-Commercial Use Only
only use Non-commercial 14th International Conference on Thalassaemia and Other Haemoglobinopathies 16th TIF Conference for Patients and Parents 17-19 November 2017 • Grand Hotel Palace, Thessaloniki, Greece only use For thalassemia patients with chronic transfusional iron overload... Make a lasting impression with EXJADENon-commercial film-coated tablets The efficacy of deferasirox in a convenient once-daily film-coated tablet Please see your local Novartis representative for Full Product Information Reference: EXJADE® film-coated tablets [EU Summary of Product Characteristics]. Novartis; August 2017. Important note: Before prescribing, consult full prescribing information. iron after having achieved a satisfactory body iron level and therefore retreatment cannot be recommended. ♦ Maximum daily dose is 14 mg/kg body weight. ♦ In pediatric patients the Presentation: Dispersible tablets containing 125 mg, 250 mg or 500 mg of deferasirox. dosing should not exceed 7 mg/kg; closer monitoring of LIC and serum ferritin is essential Film-coated tablets containing 90 mg, 180 mg or 360 mg of deferasirox. to avoid overchelation; in addition to monthly serum ferritin assessments, LIC should be Indications: For the treatment of chronic iron overload due to frequent blood transfusions monitored every 3 months when serum ferritin is ≤800 micrograms/l. (≥7 ml/kg/month of packed red blood cells) in patients with beta-thalassemia major aged Dosage: Special population ♦ In moderate hepatic impairment (Child-Pugh B) dose should 6 years and older. ♦ Also indicated for the treatment of chronic iron overload due to blood not exceed 50% of the normal dose. Should not be used in severe hepatic impairment transfusions when deferoxamine therapy is contraindicated or inadequate in the following (Child-Pugh C). -

224 Subpart H—Hematology Kits and Packages
§ 864.7040 21 CFR Ch. I (4–1–02 Edition) Subpart H—Hematology Kits and the treatment of venous thrombosis or Packages pulmonary embolism by measuring the coagulation time of whole blood. § 864.7040 Adenosine triphosphate re- (b) Classification. Class II (perform- lease assay. ance standards). (a) Identification. An adenosine [45 FR 60611, Sept. 12, 1980] triphosphate release assay is a device that measures the release of adenosine § 864.7250 Erythropoietin assay. triphosphate (ATP) from platelets fol- (a) Identification. A erythropoietin lowing aggregation. This measurement assay is a device that measures the is made on platelet-rich plasma using a concentration of erythropoietin (an en- photometer and a luminescent firefly zyme that regulates the production of extract. Simultaneous measurements red blood cells) in serum or urine. This of platelet aggregation and ATP re- assay provides diagnostic information lease are used to evaluate platelet for the evaluation of erythrocytosis function disorders. (increased total red cell mass) and ane- (b) Classification. Class I (general mia. controls). (b) Classification. Class II. The special [45 FR 60609, Sept. 12, 1980] control for this device is FDA’s ‘‘Docu- ment for Special Controls for Erythro- § 864.7060 Antithrombin III assay. poietin Assay Premarket Notification (a) Identification. An antithrombin III (510(k)s).’’ assay is a device that is used to deter- [45 FR 60612, Sept. 12, 1980, as amended at 52 mine the plasma level of antithrombin FR 17733, May 11, 1987; 65 FR 17144, Mar. 31, III (a substance which acts with the 2000] anticoagulant heparin to prevent co- agulation). This determination is used § 864.7275 Euglobulin lysis time tests. -

Fetal and Embryonic Haemoglobins P
Review Article J Med Genet: first published as 10.1136/jmg.10.1.50 on 1 March 1973. Downloaded from Journal of Medical Genetics (1973). 10, 50. Fetal and Embryonic Haemoglobins P. A. LORKIN MRC Abnormal Haemoglobin Unit, University Department of Biochemistry, Cambridge Haemoglobin has been the subject of intensive form a nearly spherical molecule with extensive research for many years and is one of the most areas of contact between unlike chains; the two thoroughly understood of all protein molecules. main types of contact are denoted alp, and alg2 The amino-acid sequences of haemoglobins from The tetramer exhibits cooperative behaviour or many species of animals have been determined haem-haem interaction. As each haem combines (tabulated by Dayhoff, 1969) and the molecular with oxygen the affinity of successive haems in- structures of horse and human haemoglobins have creases. The oxygen affinity curve of the tetramer been determined in great detail by x-ray crystallo- is sigmoidal and may be represented approximately graphy (Perutz et al, 1968a and b; Perutz 1969). A by the Hill equation:* mechanism of action of haemoglobin has been pro- = kpo2n posed (Perutz, 1970a and b and 1972). The y haemoglobins of higher organisms share a common +kpo2n tetrameric structure built up of two pairs of unlike Oxygen affinity data are usually presented in copyright. chains; the a chains containing 141 amino-acid terms of P102, the partial pressure of oxygen re- residues and the non-a chains containing generally quired to attain half saturation with oxygen, and of 145 or 146 amino acids. In man, five types of n, the exponent of the Hill equation. -

ATSDR Case Studies in Environmental Medicine Nitrate/Nitrite Toxicity
ATSDR Case Studies in Environmental Medicine Nitrate/Nitrite Toxicity Agency for Toxic Substances and Disease Registry Case Studies in Environmental Medicine (CSEM) Nitrate/Nitrite Toxicity Course: WB2342 CE Original Date: December 5, 2013 CE Expiration Date: December 5, 2015 Key • Nitrate toxicity is a preventable cause of Concepts methemoglobinemia. • Infants younger than 4 months of age are at particular risk of nitrate toxicity from contaminated well water. • The widespread use of nitrate fertilizers increases the risk of well-water contamination in rural areas. About This This educational case study document is one in a series of and Other self-instructional modules designed to increase the primary Case Studies care provider’s knowledge of hazardous substances in the in environment and to promote the adoption of medical Environmen- practices that aid in the evaluation and care of potentially tal Medicine exposed patients. The complete series of Case Studies in Environmental Medicine is located on the ATSDR Web site at URL: http://www.atsdr.cdc.gov/csem/csem.html In addition, the downloadable PDF version of this educational series and other environmental medicine materials provides content in an electronic, printable format. Acknowledgements We gratefully acknowledge the work of the medical writers, editors, and reviewers in producing this educational resource. Contributors to this version of the Case Study in Environmental Medicine are listed below. Please Note: Each content expert for this case study has indicated that there is no conflict of interest that would bias the case study content. CDC/ATSDR Author(s): Kim Gehle MD, MPH CDC/ATSDR Planners: Charlton Coles, Ph.D.; Kimberly Gehle, MD; Sharon L. -

Hereditary Spherocytosis: Clinical Features
Title Overview: Hereditary Hematological Disorders of red cell shape. Disorders Red cell Enzyme disorders Disorders of Hemoglobin Inherited bleeding disorders- platelet disorders, coagulation factor Anthea Greenway MBBS FRACP FRCPA Visiting Associate deficiencies Division of Pediatric Hematology-Oncology Duke University Health Service Inherited Thrombophilia Hereditary Disorders of red cell Disorders of red cell shape (cytoskeleton): cytoskeleton: • Mutations of 5 proteins connect cytoskeleton of red cell to red cell membrane • Hereditary Spherocytosis- sphere – Spectrin (composed of alpha, beta heterodimers) –Ankyrin • Hereditary Elliptocytosis-ellipse, elongated forms – Pallidin (band 4.2) – Band 4.1 (protein 4.1) • Hereditary Pyropoikilocytosis-bizarre red cell forms – Band 3 protein (the anion exchanger, AE1) – RhAG (the Rh-associated glycoprotein) Normal red blood cell- discoid, with membrane flexibility Hereditary Spherocytosis: Clinical features: • Most common hereditary hemolytic disorder (red cell • Neonatal jaundice- severe (phototherapy), +/- anaemia membrane) • Hemolytic anemia- moderate in 60-75% cases • Mutations of one of 5 genes (chromosome 8) for • Severe hemolytic anaemia in 5% (AR, parents ASx) cytoskeletal proteins, overall effect is spectrin • fatigue, jaundice, dark urine deficiency, severity dependant on spectrin deficiency • SplenomegalSplenomegaly • 200-300:million births, most common in Northern • Chronic complications- growth impairment, gallstones European countries • Often follows clinical course of affected -

Alpha Thalassemia Trait
Alpha Thalassemia Trait Alpha Thalassemia Trait Produced by St. Jude Children’s Research Hospital, Departments of Hematology, Patient Education, 1 and Biomedical Communications. Funds were provided by St. Jude Children’s Research Hospital, ALSAC, and a grant from the Plough Foundation. This document is not intended to replace counseling by a trained health care professional or genetic counselor. Our aim is to promote active participation in your care and treatment by providing information and education. Questions about individual health concerns or specific treatment options should be discussed with your doctor. For general information on sickle cell disease and other blood disorders, please visit our Web site at www.stjude.org/sicklecell. Copyright © 2009 St. Jude Children’s Research Hospital Alpha thalassemia trait All red blood cells contain hemoglobin (HEE muh glow bin), which carries oxygen from your lungs to all parts of your body. Alpha thalassemia (thal uh SEE mee uh) trait is a condition that affects the amount of hemo- globin in the red blood cells. • Adult hemoglobin (hemoglobin A) is made of alpha and beta globins. • Normally, people have 4 genes for alpha globin with 2 genes on each chromosome (aa/aa). People with alpha thalassemia trait only have 2 genes for alpha globin, so their bodies make slightly less hemoglobin than normal. This trait was passed on from their parents, like hair color or eye color. A trait is different from a disease 2 Alpha thalassemia trait is not a disease. Normally, a trait will not make you sick. Parents who have alpha thalassemia trait can pass it on to their children. -

Methemoglobinemia and Ascorbate Deficiency in Hemoglobin E Β Thalassemia: Metabolic and Clinical Implications
From www.bloodjournal.org by guest on April 2, 2015. For personal use only. Plenary paper Methemoglobinemia and ascorbate deficiency in hemoglobin E  thalassemia: metabolic and clinical implications Angela Allen,1,2 Christopher Fisher,1 Anuja Premawardhena,3 Dayananda Bandara,4 Ashok Perera,4 Stephen Allen,2 Timothy St Pierre,5 Nancy Olivieri,6 and David Weatherall1 1MRC Molecular Haematology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, John Radcliffe Hospital, Oxford, United Kingdom; 2College of Medicine, Swansea University, Swansea, United Kingdom; 3University of Kelaniya, Colombo, Sri Lanka; 4National Thalassaemia Centre, District Hospital, Kurunegala, Sri Lanka; 5School of Physics, University of Western Australia, Crawley, Australia; and 6Hemoglobinopathy Research, University Health Network, Toronto, ON During investigations of the phenotypic man hypoxia induction factor pathway is There was, in addition, a highly signifi- diversity of hemoglobin (Hb) E  thalasse- not totally dependent on ascorbate lev- cant correlation between methemoglobin mia, a patient was encountered with per- els. A follow-up study of 45 patients with levels, splenectomy, and factors that sistently high levels of methemoglobin HbE  thalassemia showed that methemo- modify the degree of globin-chain imbal- associated with a left-shift in the oxygen globin levels were significantly increased ance. Because methemoglobin levels are dissociation curve, profound ascorbate and that there was also a significant re- modified by several mechanisms and may deficiency, and clinical features of scurvy; duction in plasma ascorbate levels. Hap- play a role in both adaptation to anemia these abnormalities were corrected by toglobin levels were significantly re- and vascular damage, there is a strong treatment with vitamin C. -

ZNF410 Represses Fetal Globin by Devoted Control of CHD4/Nurd
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.31.272856; this version posted August 31, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Title ZNF410 represses fetal globin by devoted control of CHD4/NuRD Authors Divya S. Vinjamur1, Qiuming Yao1,2, Mitchel A. Cole1, Connor McGuckin1, Chunyan Ren1, Jing Zeng1, Mir Hossain1, Kevin Luk3, Scot A. Wolfe3, Luca Pinello2, Daniel E. Bauer1,4 1Division of Hematology/Oncology, Boston Children’s Hospital, Department of Pediatric Oncology, Dana-Farber Cancer Institute, Harvard Stem Cell Institute, Broad Institute, Department of Pediatrics, Harvard Medical School, Boston, Massachusetts 02115, USA 2Molecular Pathology Unit, Center for Cancer Research, and Center for Computational and Integrative Biology, Massachusetts General Hospital, Department of Pathology, Harvard Medical School, Boston, Massachusetts 02129, USA 3Department of Molecular, Cell and Cancer Biology, Li Weibo Institute for Rare Diseases Research, University of Massachusetts Medical School, Worcester, Massachusetts 01605, USA 4Correspondence: [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2020.08.31.272856; this version posted August 31, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Abstract Major effectors of adult-stage fetal globin silencing include the transcription factors (TFs) BCL11A and ZBTB7A/LRF and the NuRD chromatin complex, although each has potential on- target liabilities for rational �-hemoglobinopathy therapeutic inhibition. -

Analysis of Genetic Determinants Associated with Persistent Synthesis of Fetal Hemoglobin
UNIVERSITÀ DEGLI STUDI DI SASSARI PhD School in Biomolecular and Biotechnological Sciences Curriculum: Biochemistry and Molecular Biology Director: Prof. Claudia Crosio “XXVI Ciclo” Analysis of genetic determinants associated with Persistent Synthesis of Fetal Hemoglobin Supervisor: Monica Pirastru, PhD Director: Prof. Claudia Crosio PhD Student: Sandro Trova ................................................................................................................................................. INDEX INDEX ABSTRACT ................................................................................... 3 INTRODUCTION ......................................................................... 4 1. Hemoglobin .......................................................................................... 4 1.1 Structure and function of Hemoglobin ........................................ 4 1.2 Structure of globin genes and their cluster organization ............. 5 1.3 Genomic context of the α– and β–globin gene clusters .............. 9 2. Globin gene switching ....................................................................... 12 2.1 Regulatory regions and transcription factors of globin genes ... 13 2.2 The β–Globin Locus Control Region (β–LCR) role in globin expression ....................................................................... 20 2.3 Chromatin role in β–like globin gene expression: the PYR role .............................................................................. 25 2.4 Summary on the fetal to adult switch ....................................... -

Sickle Cell: It's Your Choice
Sickle Cell: It’s Your Choice What Does “Sickle Cell” Mean? Sickle is a type of hemoglobin. Hemoglobin is the substance that carries oxygen in the blood and gives blood its red color. A person’s hemoglobin type is not the same thing as blood type. The type of hemoglobin we have is determined by genes that we inherit from our parents. The majority of individuals have only the “normal” type of hemoglobin (A). However, there are a variety of other hemoglobin types. Sickle hemoglobin (S) is one of these types. There Are Two Forms of Sickle Cell. Sickle cell occurs in two forms. Sickle cell trait is not a disease; Sickle cell anemia (or sickle cell disease) is a disease. Sickle Cell Trait (or Sickle Trait) Sickle cell trait is found primarily in African Americans, people from areas around the Mediterranean Sea, and from islands in the Caribbean. Sickle cell trait occurs when a person inherits one sickle cell gene from one parent and one normal hemoglobin gene from the other parent. A person with sickle cell trait is healthy and usually is not aware that he or she has the sickle cell gene. A person who has sickle trait can pass it on to their children. If one parent has sickle cell trait and the other parent has the normal type of hemoglobin, there is a 50% (1 in 2) chance with EACH pregnancy that the baby will be born with sickle cell trait. When ONE parent has sickle cell trait, the child may inherit: • 50% chance for two normal hemoglobin genes (normal hemoglobin- AA), OR • 50% chance for one normal hemoglobin gene and one sickle cell gene (sickle cell trait- AS). -

Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases
cells Review Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases George J. Kontoghiorghes * and Christina N. Kontoghiorghe Postgraduate Research Institute of Science, Technology, Environment and Medicine, CY-3021 Limassol, Cyprus * Correspondence: [email protected]; Tel./Fax: +357-2627-2076 Received: 7 May 2020; Accepted: 5 June 2020; Published: 12 June 2020 Abstract: Iron is essential for all living organisms. Many iron-containing proteins and metabolic pathways play a key role in almost all cellular and physiological functions. The diversity of the activity and function of iron and its associated pathologies is based on bond formation with adjacent ligands and the overall structure of the iron complex in proteins or with other biomolecules. The control of the metabolic pathways of iron absorption, utilization, recycling and excretion by iron-containing proteins ensures normal biologic and physiological activity. Abnormalities in iron-containing proteins, iron metabolic pathways and also other associated processes can lead to an array of diseases. These include iron deficiency, which affects more than a quarter of the world’s population; hemoglobinopathies, which are the most common of the genetic disorders and idiopathic hemochromatosis. Iron is the most common catalyst of free radical production and oxidative stress which are implicated in tissue damage in most pathologic conditions, cancer initiation and progression, neurodegeneration and many other diseases. The interaction of iron and iron-containing proteins with dietary and xenobiotic molecules, including drugs, may affect iron metabolic and disease processes. Deferiprone, deferoxamine, deferasirox and other chelating drugs can offer therapeutic solutions for most diseases associated with iron metabolism including iron overload and deficiency, neurodegeneration and cancer, the detoxification of xenobiotic metals and most diseases associated with free radical pathology. -
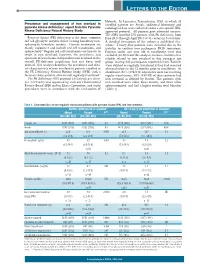
Prevalence and Management of Iron Overload in Pyruvate Kinase Deficiency
LETTERS TO THE EDITOR Helsinki. In Lancaster, Pennsylvania, USA, in which all Prevalence and management of iron overload in enrolled patients are Amish, additional laboratory and pyruvate kinase deficiency: report from the Pyruvate radiological data were collected under a site-specific IRB- Kinase Deficiency Natural History Study approved protocol. All patients gave informed consent. The NHS enrolled 278 patients with PK deficiency from Pyruvate kinase (PK) deficiency is the most common June 2014 through April 2017 at 31 centers in 6 countries. red cell glycolytic enzyme defect causing hereditary non- A detailed description of the cohort is published else- spherocytic hemolytic anemia. Current treatments are where.2 Twenty-four patients were excluded due to the mainly supportive and include red cell transfusions and inability to confirm two pathogenic PKLR mutations. splenectomy.1 Regular red cell transfusions are known to Patients under one year old at enrollment were also result in iron overload; however, the prevalence and excluded (n=12) from this analysis, because ferritin is less spectrum of transfusion-independent iron overload in the reliably related to iron overload in this youngest age overall PK-deficient population has not been well group, leaving 242 participants reported herein. Patients defined. This analysis describes the prevalence and clini- were defined as regularly transfused if they had received cal characteristics of iron overload in patients enrolled in ≥6 transfusions in the 12 months prior to enrollment. At the PK Deficiency Natural History Study (NHS) with a enrollment, 82% (198/242) of patients were not receiving focus on those patients who are not regularly transfused.2 regular transfusions; 38% (53/138) of these patients had The PK deficiency NHS protocol (clinicaltrials.gov identi- iron overload as defined by ferritin.