Urinary Sample Collection Methods in Ileal Conduit Urinary Diversion Patients a Randomized Control Trial Markku H
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Continent Urostomy Guide
$POUJOFOU6SPTUPNZ(VJEF "QVCMJDBUJPOPGUIF6OJUFE0TUPNZ"TTPDJBUJPOTPG"NFSJDB *OD i4FJ[FUIF 0QQPSUVOJUZw CONTINENT UROSTOMY GUIDE Ilene Fleischer, MSN, RN, CWOCN, Author Patti Wise, BSN, RN, CWOCN, Author Reviewed by: Authors and Victoria A.Weaver, RN, MSN, CETN Revised 2009 by Barbara J. Hocevar, BSN,RN,CWOCN, Manager, ET/WOC Nursing, Cleveland Clinic © 1985 Ilene Fleischer and Patti Wise This guidebook is available for free, in electronic form, from United Ostomy Associations of America (UOAA). UOAA may be contacted at: www.ostomy.org • [email protected] • 800-826-0826 CONTENTS INTRODUCTION . 3 WHAT IS A CONTINENT UROSTOMY? . 4 THE URINARY TRACT . 4 BEFORE THE SURGERY . .5 THE SURGERY . .5 THE STOMA . 7 AFTER THE SURGERY . 7 Irrigation of the catheter(s) 8 Care of the drainage receptacles 9 Care of the stoma 9 Other important information 10 ROUTINE CARE AT HOME . 10 Catheterization schedule 11 How to catheterize your pouch 11 Special considerations when catheterizing 11 Care of the catheter 12 Other routine care 12 HELPFUL HINTS . .13 SUPPLIES FOR YOUR CONTINENT UROSTOMY . 14 LIFE WITH YOUR CONTINENT UROSTOMY . 15 Clothing 15 Diet 15 Activity and exercise 15 Work 16 Travel 16 Telling others 17 Social relationships 17 Sexual relations and intimacy 17 RESOURCES . .19 GLOSSARY OF TERMS . 20 BIBLIOGRAPHY . .21 1 INTRODUCTION Many people have ostomies and lead full and active lives. Ostomy surgery is the main treatment for bypassing or replacing intestinal or urinary organs that have become diseased or dysfunctional. “Ostomy” means opening. It refers to a number of ways that bodily wastes are re-routed from your body. A urostomy specifi cally redirects urine. -
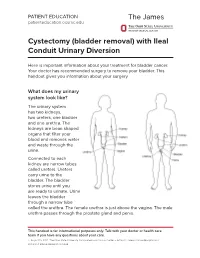
Cystectomy (Bladder Removal) with Ileal Conduit Urinary Diversion
PATIENT EDUCATION patienteducation.osumc.edu Cystectomy (bladder removal) with Ileal Conduit Urinary Diversion Here is important information about your treatment for bladder cancer. Your doctor has recommended surgery to remove your bladder. This handout gives you information about your surgery. What does my urinary system look like? The urinary system has two kidneys, two ureters, one bladder and one urethra. The kidneys are bean shaped organs that filter your blood and removes water and waste through the urine. Connected to each kidney are narrow tubes called ureters. Ureters carry urine to the bladder. The bladder stores urine until you are ready to urinate. Urine leaves the bladder through a narrow tube called the urethra. The female urethra is just above the vagina. The male urethra passes through the prostate gland and penis. This handout is for informational purposes only. Talk with your doctor or health care team if you have any questions about your care. © August 19, 2021. The Ohio State University Comprehensive Cancer Center – Arthur G. James Cancer Hospital and Richard J. Solove Research Institute. Cystectomy (bladder removal) with Ileal Conduit Urinary Diversion A cystectomy is surgery to remove the bladder. In men, the prostate, seminal vesicles and lymph nodes may also be removed. In women, the ovaries, fallopian tubes, uterus, cervix, vagina, urethra and lymph nodes may be removed. Your doctor will talk with you about the surgery and what is best for you. After the bladder is removed, your doctor will make a new urinary system. One option is an ileal conduit, also called a urostomy. This will direct your urine to drain through a small opening in your abdomen (belly) called a “stoma”. -

1 an Ileal Conduit Urinary Diversion Some Practical Questions
An Ileal Conduit Urinary Diversion Some practical questions & answers with Nancy, a bladder cancer survivor with an Ileal Conduit. An ileal conduit is a system of urinary drainage which a surgeon creates using the small intestine after removing the bladder. To do this, the surgeon takes a short segment of the small intestine and places it at an opening he has made on the surface of the abdomen to create a mouth, or stoma. The ureters, which normally carry urine from the kidneys to the bladder, are then attached to the other end of the segment of intestine. The urine now travels from the kidneys, via the ureters and the newly formed ileal conduit, to the stoma and out into a collecting pouch known as an ostomy bag (or urostomy.) This is worn outside the body around the stoma 24 hours a day. Because the nerves and blood supply are preserved, the new conduit is able to propel the urine out of the body and into the urostomy. Tell us about Recuperation after the Surgery Nancy: One of the advantages of the ileal conduit is that it requires less surgical time than other, more complex, diversions. This procedure was developed during the 1940’s and is still the most used technique for urinary diversion. When I was released from the hospital, I came home with no tubes or catheters. I had no diet restrictions because only a small part of the intestine is used. However, I understand many doctors will recommend a low residue diet for the first few weeks post op. -

Urinary Diversion - Formation of a Urostomy, Indiana Pouch and Ileal Neobladder
UROLOGIC Limited Urinary Diversion - Formation of a Urostomy, Indiana Pouch and Ileal Neobladder A Guide for Patients Rotorua Text Compiled by: Michael D. Cresswell F.R.A.C.S (Urol) Angela Hewitt RCN Tauranga Urology Nurse Specialist Mark R. Fraundorfer F.R.A.C.S (Urol) • Peter J. Gilling F.R.A.C.S (Urol) • Andre M. Westenberg F.R.A.C.S (Urol) Copyright © 2001 PO Box 893, Tauranga, New Zealand, Phone: 07 578 8011 / 0800 808 870, Fax: 07 578 5038 Hamilton Patrick R. Bary F.R.A.C.S (Urol) • Michael A. Holmes F.R.A.C.S (Urol) • William L. Wright F.R.A.C.S (Urol) PO Box 19210, Hamilton, New Zealand, Phone: 07 839 8947 / 0800 923 923, Fax: 07 839 8990 URINARY DIVERSION – FORMATION OF A • UROSTOMY (ILEAL CONDUIT) • INDIANA POUCH • ILEAL NEOBLADDER This information is designed to help you, your family and friends prepare for your surgery. It will also help you plan how to take care of yourself in the weeks following your discharge from hospital. WHAT IS A URINARY DIVERSION AND WHY IS IT NEEDED? A urinary diversion is an operation performed to divert urine away from the bladder. Urine is made by the kidneys and drains down the ureters and is stored in the bladder until it is convenient for the person to empty it. There are several reasons why a urinary diversion may be needed, these include: • If a person develops cancer of the bladder or uncontrolled bleeding from the bladder after radiotherapy or some drugs, sometimes the only way to stop the bleeding or effectively rid the body of cancer is to operate and remove the bladder. -

Patient-Guidebook-Radical-Cystectomy-Greyscale-EN-May-2016.Pdf
BLADDER CANCER PATIENT GUIDEBOOK FOR PATIENTS FACING RADICAL CYSTECTOMY WHAT’S IN THIS YOU ARE GUIDEBOOK? NOT ALONE What causes This guidebook was created by people just like you – bladder cancer bladder cancer? . 3 patients and their caregivers . It is designed to offer you support, encouragement and tips on how to deal with the procedures and Muscle-invasive bladder cancer . 3 treatments for your bladder cancer . It can help you understand your disease and what to expect, the treatments you may be offered and how Understanding how to manage your disease in the years ahead . the body works . 3 The urinary system . 3 We are not medical professionals and you should always turn to your medical team for advice first . But we have been where you are now and The intestinal system . 4 have experienced what you are feeling . Staging and grading your bladder cancer . .4 This guidebook will talk about common approaches to treating muscle- invasive bladder cancer (MIBC), but keep in mind that there can be some How will my cancer differences, depending on the practices of your medical team and on be treated? . 4 your specific situation . We will not be using a lot of medical language, Questions to ask except where it might help you better communicate with your medical your surgeon . 5 team and understand your disease . You’ll find a handy glossary of terms The bladder is gone – on Bladder Cancer Canada’s website at now what? . 5 https://bladdercancercanada.org/en/facing-bladder-cancer/glossary-of- terms . Ileal Conduit (Ostomy) . 6 Orthotopic Neobladder . -

Incontinent Urostomy
Good Practice in Health Care European Association Incontinent of Urology Nurses PO Box 30016 6803 AA Arnhem The Netherlands Urostomy T +31 (0)26 389 0680 F +31 (0)26 389 0674 [email protected] www.eaun.uroweb.org 2009 European European Association Association of Urology of Urology Nurses Nurses Good Practice in Health Care Incontinent Urostomy V. Geng H. Cobussen-Boekhorst S. Fillingham S. Holroyd B. Kiesbye S. Vahr Introduction The European Association of Urology Nurses (EAUN) was created in April 2000 to represent European urological nurses. The EAUN’s underlying goal is to foster the highest standards of urological nursing care throughout Europe. With administrative, financial and advisory support from the European Association of Urology (EAU), the EAUN also encourages research and aspires to develop European standards for education and accreditation of urology nurses. We believe that excellent healthcare goes beyond geographical boundaries. Improving current standards of urological nursing care has been top of our agenda, with the aim of directly helping our members develop or update their expertise. To fulfil this essential goal, we are publishing the latest addition to our Good Practice in Health Care series, a comprehensive compilation of theoretical knowledge and practical guidelines on incontinent urostomy. Although there is considerable literature on incontinent urinary diversion (ileal conduit or urostomy), to our knowledge prior to this publication there was only limited evidence-based guidance for nurses available on this topic. The EAUN Guidelines Group believes there is a need to provide guidelines clearly stating the level of evidence of each procedure and recommendation with the aim of improving current practices and delivering a standard and reliable protocol. -

New Ostomy Patient Guide 2020
NewNew OstomyOstomy PatientPatient GuideGuide • Colostomy • Ileostomy • Urostomy • Continent Diversion A publication of Find A Local Support Group www.ostomy.org or call 800-826-0826 “I felt like I had tried every pouch on the market, then someone mentioned Nu-Hope. Ever since, I’ve been able to get out of bed and back on the golf course!” Never miss the moment Nu-Hope founder Edmund Galindo believed there had to be a way to get back to the links after his ostomy. Playing golf was one activity he couldn’t live without. After many experiments, Ed created a new kind of ostomy pouch and support belt that allowed him to get back swinging. “Just when you think there is no hope, there’s Nu-Hope!” Download Belt Guide: bit.ly/NuHopeBeltGuide nu-hope.com fb.com/nuhopelabs ® 1.800.899.5017 @nuhopelabs [email protected] LABORATORIES, INC. NewNew OstomyOstomy PatientPatient GuideGuide Ostomy Basics 6 What is an Ostomy? By Cliff Kalibjian 12 Ostomy A to Z By Cliff Kalibjian, Revised by Joanna Burgess-Stocks, BSN, RN, CWOCN 18 A Successful Recovery By Diana Gallagher, MS, RN, CWOCN, CFCN 20 Ostomy Reversals By David E. Beck, MD, FACS, FASCRS 22 Peristomal Hernias By David E. Beck, MD, FACS, FASCRS 24 Peristomal Skin Care By Joan Junkin, MSN, APRN-CNS, CWOCN Product Guide 26 One-Piece Vs. Two-Piece Adapted from Pouching Systems Patient Educational Sheet 28 Five Reasons Pouches Leak By Anita Prinz, RN, MSN, CWOCN 32 Peristomal Skin Products By Joy Boarini, MSN, WOCN Colostomy Care 36 Basic Colostomy Care By Leslie Washuta, RN, BSN, CWON 43 Colostomy Irrigation By Ian Settlemire, The Phoenix Magazine Editor 46 Beautiful Butterfly By Charles Redner Table of Contents Continues New Ostomy Ileostomy Care Patient Guide A publication of 48 Basic Ileostomy Care By Leslie Washuta, RN, BSN, CWON 54 The Pilates Pro By Ryan Hodgkinson 56 Short Bowel Syndrome By United Ostomy Associations of America This publication is funded by the nonprofit United Ostomy Urostomy Care Associations of America and by advertising. -

(A Bladder Chimney) for the Treatment of Severe Neurogenic Vesical Dysfunction
Paraplegia (1995) 33, 530-535 © 1995 International Medical Society of Paraplegia All rights reserved 0031-1758/95 $12.00 Cutaneous ileocystostomy (a bladder chimney) for the treatment of severe neurogenic vesical dysfunction DA Rivas 1 , S Karasick2 and MB Chancellor1 2 1 Assistant Professor of Urology, Department of Urology, Clinical Professor of Radiology, Department of Radiology, Jefferson Medical College, Philadelphia, PA 19107, USA The aim of this study was to investigate the efficacy and morbidity of cutaneous ileo cystostomy, as an alternative to cystectomy and ileal conduit urinary diversion, for patients with end-stage neurogenic vesical dysfunction. Three male and eight female patients, mean age 41 years (range 28-59), with a mean duration of a neuropathic bladder of 8 years (range 4-17 years) underwent evaluation for ileocystostomy urinary diversion. Indications for the procedure included a bladder capacity � 200 ml (10 patients), recurrent febrile urinary tract infection (nine patients), and urinary incontinence despite an indwelling urethral catheter (all eight women). Each was felt to be a poor candidate for, or refused, continent urinary diversion or bladder augmentation cystoplasty. All eight females required concomitant pubovaginal sling urethral compression to eliminate urinary leakage from a patulous, non-functional urethra. Two patients required bilateral ureteral reimplantation for grade III-IVIV reflux. Effective low-pressure urinary stomal drainage was achieved without the need for chronic catheterization in all of the patients with a mean duration of follow-up of 24 months (range 6-60 months). No patient has developed pyelonephritis since the procedure. Urethral urinary leakage was eliminated in all of the female patients, whilst vesicoureteral reflux resolved in those with reflux preoperatively. -

Urostomy Guide
6SPTUPNZ(VJEF "QVCMJDBUJPOPGUIF6OJUFE0TUPNZ"TTPDJBUJPOTPG"NFSJDB *OD A Message To You... Urostomy surgery is a lifesaving surgery that enables a person to enjoy a full range of activities, including traveling, sports, family life and work. Thousands of people annually undergo ostomy surgery for various reasons and return to a healthy, functioning lifestyle. The United Ostomy Associations of America (UOAA) is a volunteer organization dedicated to helping those who have or will have ostomy or other diversionary surgery by providing one-on-one support, local support group meetings, conferences, and educational material through its web site, printed material and The Phoenix magazine. You have many peers in the UOAA who are ready to answer your questions, provide support and reassure you that you can have a full, productive life after ostomy surgery. We invite you to join us as we fulfill our mission in helping others. From the United Ostomy Associations of America UROSTOMY GUIDE Reviewed by Jane Fellows, RN, CWOCN 2017 Previously reviewed by: Nancy Gutman, RN, CWOCN 2011, Jan Clark, RNET, CWOCN Helen DuBois, RNET This guidebook is available for free, in electronic form, from the United Ostomy Associations of America (UOAA). It was originally produced, copyrighted and sold by the United Ostomy Association (UOA), the national US ostomy organization from 1962 to 2005, which released its copyrights on this material. UOAA may be contacted at: www.ostomy.org • [email protected] • 800-826-0826 CONTENTS INTRODUCTION . 2 FACTS ABOUT UROSTOMIES . .3 NORMAL URINARY SYSTEM . 4 CONVENTIONAL UROSTOMY . 4 CONTINENT UROSTOMIES . .5 UROSTOMY MANAGEMENT . 5 Pouching Systems 5 Skin Protection 6 Changing the Pouching System 7 Emptying the Pouch 7 Belts and Tape 8 Night Drainage System 8 Ostomy Supplies 9 HELPFUL HINTS . -

Understanding Bladder Cancer
About the Bladder The bladder is a hollow, round-shaped, muscular organ that stores urine. The urine is made in the kidneys and flows through tube-like structures called ureters that connect to the bladder. The urine then passes through the urethra during the process of urination. The bladder muscle assists in the urination process by contracting (squeezing) to help remove the urine out of the bladder. In women the urethra is very short. In men the urethra is longer and passes through the prostate gland to the tip of the penis. The Urinary System The wall of the bladder is made of layers. A thin surface layer called the urothelium lines the inside of the bladder. Next is a layer of loose connective tissue called the lamina propria. Covering the lamina propria is the bladder muscle. Outside of the bladder is a layer of fat. The wall of the bladder is made of layers. Cancer begins in the lining layer and grows into the bladder wall. As the cancer grows through the layers into the wall of the bladder, it becomes harder to treat. The inside of the bladder is lined with a layer of cells called urothelial cells. The same type of cells also line the kidneys, the ureters (tubes connecting the kidneys to the bladder), and the urethra. Cancer can start in the lining cells in any of these parts of the urinary system. Types of Bladder Cancer Bladder tumors are grouped by the way the cancer cells look under a microscope. The type of bladder cancer you have can affect your treatment options because different types respond to different treatments. -
![Download This Topic [PDF]](https://docslib.b-cdn.net/cover/3513/download-this-topic-pdf-13383513.webp)
Download This Topic [PDF]
cancer.org | 1.800.227.2345 Urostomy Guide Urostomy surgery is needed when the bladder isn’t working the way it should. There are 4 major bladder problems that may be treated with a urostomy: ● Bladder cancer ● Damage to the nerves that control the bladder (called neurogenic bladder disease) ● Birth defects ● Chronic inflammation of the bladder For the thousands of people who have serious bladder diseases, a urostomy can be the start of a new and healthier life. If you have had a chronic (long-term) problem or a life- threatening disease like bladder cancer, you can look forward to feeling better after you recover from urostomy surgery. You can also look forward to returning to most, if not all of the activities you enjoyed in the past. This guide will help you better understand urostomy – what it is, why it’s needed, how it affects the normal urinary system, and the changes it can bring to a person’s life. ● What Is a Urostomy? ● Types of Urostomies and Pouching Systems ● Caring for a Urostomy What Is a Urostomy? 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 Aurostomy is an opening in the belly (abdominal wall) that’s made during surgery. It re- directs urine away from a bladder that’s diseased, has been injured, or isn't working as it should. The bladder is either bypassed or removed. (Surgery to remove the bladder is called a cystectomy.) After this surgery, urine is passed out of the body through an opening on the belly called a stoma. A Wound Ostomy Continence nurse (WOCN or WOC nurse)or the surgeon will figure out the best location for your stoma. -

Radical Cystectomy and Indiana Pouch: a Patient's Guide
Urology The Experts in Urologic Care Radical Cystectomy and Indiana Pouch: A Patient’s Guide Urology 2 The Experts in Urologic Care Table of Contents Detecting And Diagnosing Bladder Cancer What Exactly Is The “Urinary System”? For Men ...................................................................................................................................................4 For Women ..............................................................................................................................................5 What Is Bladder Cancer? .................................................................................................................................6 Treatment For Bladder Cancer .........................................................................................................................7 What Research Is Being Done On Bladder Cancer? ..........................................................................................8 What Is Radical Cystectomy With Indiana Pouch? Anatomy Of Radical Cystectomy With Indiana Pouch For Men ...................................................................................................................................................9 For Women ..............................................................................................................................................9 What Is An Indiana Pouch? ...........................................................................................................................9 What Care Will Be