Pseudomys Shortridgei) in Lake Magenta Nature Reserve
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of Plants Used by Carnaby's Black Cockatoo
Plants Used by Carnaby's Black Cockatoo List prepared by Christine Groom, Department of Environment and Conservation 15 April 2011 For more information on plant selection or references used to produce this list please visit the Plants for Carnaby's Search Tool webpage at www.dec.wa.gov.au/plantsforcarnabys Used for Soil type Soil drainage Priority for planting Sun Species Growth form Flower colour Origin for exposure Carnaby's Feeding Nesting Roosting Clayey Gravelly Loamy Sandy drained Well drained Poorly Waterlogged affected Salt Acacia baileyana (Cootamundra wattle)* Low Tree Yellow Australian native Acacia pentadenia (Karri Wattle) Low Tree Cream WA native Acacia saligna (Orange Wattle) Low Tree Yellow WA native Agonis flexuosa (Peppermint Tree) Low Tree White WA native Araucaria heterophylla (Norfolk Island Pine) Low Tree Green Exotic to Australia Banksia ashbyi (Ashby's Banksia) Medium Tree or Tall shrub Yellow, Orange WA native Banksia attenuata (Slender Banksia) High Tree Yellow WA native Banksia baxteri (Baxter's Banksia) Medium Tall shrub Yellow WA native Banksia carlinoides (Pink Dryandra) Medium Medium or small shrub White, cream, pink WA native Banksia coccinea (Scarlet Banksia) Medium Tree Red WA native Banksia dallanneyi (Couch Honeypot Dryandra) Low Medium or small shrub Orange, brown WA native Banksia ericifolia (Heath-leaved Banksia) Medium Tall shrub Orange Australian native Banksia fraseri (Dryandra) Medium Medium or small shrub Orange WA native Banksia gardneri (Prostrate Banksia) Low Medium -

Platypus Collins, L.R
AUSTRALIAN MAMMALS BIOLOGY AND CAPTIVE MANAGEMENT Stephen Jackson © CSIRO 2003 All rights reserved. Except under the conditions described in the Australian Copyright Act 1968 and subsequent amendments, no part of this publication may be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, mechanical, photocopying, recording, duplicating or otherwise, without the prior permission of the copyright owner. Contact CSIRO PUBLISHING for all permission requests. National Library of Australia Cataloguing-in-Publication entry Jackson, Stephen M. Australian mammals: Biology and captive management Bibliography. ISBN 0 643 06635 7. 1. Mammals – Australia. 2. Captive mammals. I. Title. 599.0994 Available from CSIRO PUBLISHING 150 Oxford Street (PO Box 1139) Collingwood VIC 3066 Australia Telephone: +61 3 9662 7666 Local call: 1300 788 000 (Australia only) Fax: +61 3 9662 7555 Email: [email protected] Web site: www.publish.csiro.au Cover photos courtesy Stephen Jackson, Esther Beaton and Nick Alexander Set in Minion and Optima Cover and text design by James Kelly Typeset by Desktop Concepts Pty Ltd Printed in Australia by Ligare REFERENCES reserved. Chapter 1 – Platypus Collins, L.R. (1973) Monotremes and Marsupials: A Reference for Zoological Institutions. Smithsonian Institution Press, rights Austin, M.A. (1997) A Practical Guide to the Successful Washington. All Handrearing of Tasmanian Marsupials. Regal Publications, Collins, G.H., Whittington, R.J. & Canfield, P.J. (1986) Melbourne. Theileria ornithorhynchi Mackerras, 1959 in the platypus, 2003. Beaven, M. (1997) Hand rearing of a juvenile platypus. Ornithorhynchus anatinus (Shaw). Journal of Wildlife Proceedings of the ASZK/ARAZPA Conference. 16–20 March. -

Calaby References
Abbott, I.J. (1974). Natural history of Curtis Island, Bass Strait. 5. Birds, with some notes on mammal trapping. Papers and Proceedings of the Royal Society of Tasmania 107: 171–74. General; Rodents; Abbott, I. (1978). Seabird islands No. 56 Michaelmas Island, King George Sound, Western Australia. Corella 2: 26–27. (Records rabbit and Rattus fuscipes). General; Rodents; Lagomorphs; Abbott, I. (1981). Seabird Islands No. 106 Mondrain Island, Archipelago of the Recherche, Western Australia. Corella 5: 60–61. (Records bush-rat and rock-wallaby). General; Rodents; Abbott, I. and Watson, J.R. (1978). The soils, flora, vegetation and vertebrate fauna of Chatham Island, Western Australia. Journal of the Royal Society of Western Australia 60: 65–70. (Only mammal is Rattus fuscipes). General; Rodents; Adams, D.B. (1980). Motivational systems of agonistic behaviour in muroid rodents: a comparative review and neural model. Aggressive Behavior 6: 295–346. Rodents; Ahern, L.D., Brown, P.R., Robertson, P. and Seebeck, J.H. (1985). Application of a taxon priority system to some Victorian vertebrate fauna. Fisheries and Wildlife Service, Victoria, Arthur Rylah Institute of Environmental Research Technical Report No. 32: 1–48. General; Marsupials; Bats; Rodents; Whales; Land Carnivores; Aitken, P. (1968). Observations on Notomys fuscus (Wood Jones) (Muridae-Pseudomyinae) with notes on a new synonym. South Australian Naturalist 43: 37–45. Rodents; Aitken, P.F. (1969). The mammals of the Flinders Ranges. Pp. 255–356 in Corbett, D.W.P. (ed.) The natural history of the Flinders Ranges. Libraries Board of South Australia : Adelaide. (Gives descriptions and notes on the echidna, marsupials, murids, and bats recorded for the Flinders Ranges; also deals with the introduced mammals, including the dingo). -
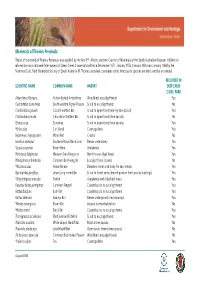
Mammals of Fleurieu Peninsula This List of Mammals of Fleurieu Peninsula Was Applied by the Late P.F
Mammals of Fleurieu Peninsula This list of mammals of Fleurieu Peninsula was applied by the late P.F. Aitken, onetime Curator of Mammals at the South Australian Museum. Additional information was obtained from surveys of Deep Creek Conservation Park in December 1971, January 1972, January 1980 and January 1984 by the Mammal Club, Field Naturalists Society of South Australia (R. Thomas, personal communication). Introduced species are indicated by an asterisk. RECORDED IN SCIENTIFIC NAME COMMON NAME HABITAT DEEP CREEK CONS. PARK Antechinus Flavipes Yellow-footed Antechinus Woodland, eucalypt forest Yes Cercartetus concinnus South western Pigmy Possum Scrub to eucalypt forest No Chalinolobus gouldii Gould's wattled Bat Scrub to open-forest (mainly tree spouts) Yes Chalinolobus moria Chocolate Wattled Bat Scrub to open-forest (tree spouts) No Eptesicus sp. Eptesicus Scrub to open-forest (tree spouts) Yes *Felis calus Cat (feral) Cosmopolitan Yes Hydromys chrysogaster Water Rat Creeks No Isoodon obesulus Southern Brown Bandicoot Dense understorey Yes *Lepus capensis Brown Hare Grasslands Yes Macropus fuliginosus Western Grey Kangaroo Heath to eucalypt forest Yes Miniopterus schreibersii Common Bent-wing Ba Eucalypt forest (caves) No *Mus musculus House Mouse Disturbed areas and early fire succession Yes Nyctophilus geoffroy Lesser Long-eared Bat Scrub to forest, semi cleared pasture (tree spouts, buildings) Yes *Oryctolagus cuniculus Rabbit Grasslands and disturbed areas Yes Pseudocheirus peregrinus Common Ringtail Coastal scrub to eucalypt -

Quaternary Murid Rodents of Timor Part I: New Material of Coryphomys Buehleri Schaub, 1937, and Description of a Second Species of the Genus
QUATERNARY MURID RODENTS OF TIMOR PART I: NEW MATERIAL OF CORYPHOMYS BUEHLERI SCHAUB, 1937, AND DESCRIPTION OF A SECOND SPECIES OF THE GENUS K. P. APLIN Australian National Wildlife Collection, CSIRO Division of Sustainable Ecosystems, Canberra and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) K. M. HELGEN Department of Vertebrate Zoology National Museum of Natural History Smithsonian Institution, Washington and Division of Vertebrate Zoology (Mammalogy) American Museum of Natural History ([email protected]) BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 341, 80 pp., 21 figures, 4 tables Issued July 21, 2010 Copyright E American Museum of Natural History 2010 ISSN 0003-0090 CONTENTS Abstract.......................................................... 3 Introduction . ...................................................... 3 The environmental context ........................................... 5 Materialsandmethods.............................................. 7 Systematics....................................................... 11 Coryphomys Schaub, 1937 ........................................... 11 Coryphomys buehleri Schaub, 1937 . ................................... 12 Extended description of Coryphomys buehleri............................ 12 Coryphomys musseri, sp.nov.......................................... 25 Description.................................................... 26 Coryphomys, sp.indet.............................................. 34 Discussion . .................................................... -

Main Roads Western Australia Report for Brand Highway Upgrade at 61.50 to 63.40 SLK Environmental Impact Assessment
Main Roads Western Australia Report for Brand Highway Upgrade at 61.50 to 63.40 SLK Environmental Impact Assessment March 2007 FINAL DRAFT Contents Executive Summary i 1. Introduction 1 1.1 Background 1 1.2 Study Area 1 1.3 Field Survey Scope 1 1.4 Purpose of this Document 2 2. Project Details 3 2.1 Proposal in a Regional Context 3 2.2 Description of the project 3 2.3 Need for the Proposal 4 2.4 Proposal Schedule 4 3. Existing Environment 5 3.1 Climate 5 3.2 Geology and Soils 5 3.3 Rivers and Wetlands 6 3.4 Groundwater 6 3.5 Reserves and Conservation Areas 6 3.6 Contaminated Sites 6 3.7 Flora and Vegetation 7 3.8 Fauna 14 4. Social Environment 17 4.1 Surrounding Land Use 17 4.2 Aboriginal Heritage 17 4.3 European Heritage 17 5. Environmental Aspects 18 6. Environmental Impacts and Management 20 6.1 Flora and Vegetation 20 6.2 Fauna 24 6.3 Drainage 28 6.4 Groundwater 28 6.5 Dust 28 6.6 Aboriginal Heritage 28 6.7 Public Safety 28 6.8 Fire Management 29 6.9 Hydrocarbon and Chemical Storage 29 6.10 Waste Management 29 7. Environmental Management Plan 30 7.1 Environmental Monitoring and Compliance 30 8. Consultation 31 9. Conclusions 32 10. Limitations 34 10.1 Survey Limitations 34 10.2 Report Limitations 34 11. References 35 Table Index Table 1 Key Characteristics of the Upgrades 3 Table 2 Climate Readings at Closest Weather Stations (BOM, 2007) 5 Table 3 Government of Western Australia (2000) Vegetation Condition Scale 9 Table 4 Vegetation extent and status for Heddle, et al. -

Natural History of the Eutheria
FAUNA of AUSTRALIA 35. NATURAL HISTORY OF THE EUTHERIA P. J. JARMAN, A. K. LEE & L. S. HALL (with thanks for help to J.H. Calaby, G.M. McKay & M.M. Bryden) 1 35. NATURAL HISTORY OF THE EUTHERIA 2 35. NATURAL HISTORY OF THE EUTHERIA INTRODUCTION Unlike the Australian metatherian species which are all indigenous, terrestrial and non-flying, the eutherians now found in the continent are a mixture of indigenous and exotic species. Among the latter are some intentionally and some accidentally introduced species, and marine as well as terrestrial and flying as well as non-flying species are abundantly represented. All the habitats occupied by metatherians also are occupied by eutherians. Eutherians more than cover the metatherian weight range of 5 g–100 kg, but the largest terrestrial eutherians (which are introduced species) are an order of magnitude heavier than the largest extant metatherians. Before the arrival of dingoes 4000 years ago, however, none of the indigenous fully terrestrial eutherians weighed more than a kilogram, while most of the exotic species weigh more than that. The eutherians now represented in Australia are very diverse. They fall into major suites of species: Muridae; Chiroptera; marine mammals (whales, seals and dugong); introduced carnivores (Canidae and Felidae); introduced Leporidae (hares and rabbits); and introduced ungulates (Perissodactyla and Artiodactyla). In this chapter an attempt is made to compare and contrast the main features of the natural histories of these suites of species and, where appropriate, to comment on their resemblance to or difference from the metatherians. NATURAL HISTORY Ecology Diet. The native rodents are predominantly omnivorous. -

Inventory of Taxa for the Fitzgerald River National Park
Flora Survey of the Coastal Catchments and Ranges of the Fitzgerald River National Park 2013 Damien Rathbone Department of Environment and Conservation, South Coast Region, 120 Albany Hwy, Albany, 6330. USE OF THIS REPORT Information used in this report may be copied or reproduced for study, research or educational purposed, subject to inclusion of acknowledgement of the source. DISCLAIMER The author has made every effort to ensure the accuracy of the information used. However, the author and participating bodies take no responsibiliy for how this informrion is used subsequently by other and accepts no liability for a third parties use or reliance upon this report. CITATION Rathbone, DA. (2013) Flora Survey of the Coastal Catchments and Ranges of the Fitzgerald River National Park. Unpublished report. Department of Environment and Conservation, Western Australia. ACKNOWLEDGEMENTS The author would like to thank many people that provided valable assistance and input into the project. Sarah Barrett, Anita Barnett, Karen Rusten, Deon Utber, Sarah Comer, Charlotte Mueller, Jason Peters, Roger Cunningham, Chris Rathbone, Carol Ebbett and Janet Newell provided assisstance with fieldwork. Carol Wilkins, Rachel Meissner, Juliet Wege, Barbara Rye, Mike Hislop, Cate Tauss, Rob Davis, Greg Keighery, Nathan McQuoid and Marco Rossetto assissted with plant identification. Coralie Hortin, Karin Baker and many other members of the Albany Wildflower society helped with vouchering of plant specimens. 2 Contents Abstract .............................................................................................................................. -

Ba3444 MAMMAL BOOKLET FINAL.Indd
Intot Obliv i The disappearing native mammals of northern Australia Compiled by James Fitzsimons Sarah Legge Barry Traill John Woinarski Into Oblivion? The disappearing native mammals of northern Australia 1 SUMMARY Since European settlement, the deepest loss of Australian biodiversity has been the spate of extinctions of endemic mammals. Historically, these losses occurred mostly in inland and in temperate parts of the country, and largely between 1890 and 1950. A new wave of extinctions is now threatening Australian mammals, this time in northern Australia. Many mammal species are in sharp decline across the north, even in extensive natural areas managed primarily for conservation. The main evidence of this decline comes consistently from two contrasting sources: robust scientifi c monitoring programs and more broad-scale Indigenous knowledge. The main drivers of the mammal decline in northern Australia include inappropriate fi re regimes (too much fi re) and predation by feral cats. Cane Toads are also implicated, particularly to the recent catastrophic decline of the Northern Quoll. Furthermore, some impacts are due to vegetation changes associated with the pastoral industry. Disease could also be a factor, but to date there is little evidence for or against it. Based on current trends, many native mammals will become extinct in northern Australia in the next 10-20 years, and even the largest and most iconic national parks in northern Australia will lose native mammal species. This problem needs to be solved. The fi rst step towards a solution is to recognise the problem, and this publication seeks to alert the Australian community and decision makers to this urgent issue. -

Bindaring Park Bassendean - Fauna Assessment
Bindaring Park Bassendean - Fauna Assessment Wetland habitat within Bindaring Park study area (Rob Browne-Cooper) Prepared for: Coterra Environment Level 3, 25 Prowse Street, WEST PERTH, WA 6005 Prepared by: Robert Browne-Cooper and Mike Bamford M.J. & A.R. Bamford Consulting Ecologists 23 Plover Way KINGSLEY WA 6026 6th April 2017 Bindaring Park - Fauna Assessment Summary Bamford Consulting Ecologists was commissioned by Coterra Environment to conduct a Level 1 fauna assessment (desktop review and site inspection) of Bindaring Park in Bassendean (the study area). The fauna survey is required to provide information on the ecological values for the Town of Bassendean’s Stage 2 Bindaring Wetland Concept Plan Development. This plan include developing design options (within wetland area) to enhance ecological values and habitat. The purpose of this report is to provide information on the fauna values of the habitat, particularly for significant species, and an overview of the ecological function of the site within the local and regional context. This assessment focuses on vertebrate fauna associated with the wetland and surrounding parkland vegetation within the study area, with consideration for connectivity with the Swan River. An emphasis is placed on locally-occurring conservation significant species and their habitat. Relevant species include Carnaby’s Black-Cockatoo, Forest Red-tailed Black-Cockatoo, and other local native species such as the Water Rat or Rakali. The fauna investigations were based on a desktop assessment and a field survey conducted in February 2017. The desktop study identified 180 vertebrate fauna species as potentially occurring in the Bindaring Park study area (see Table 3 and Appendix 5): five fish, 6 frogs, 20 reptiles, 134 birds, 8 native and 7 introduced mammals. -

Vertebrate Monitoring and Re-Sampling in Kakadu National Park
Vertebrate monitoring and re-sampling in Kakadu National Park Final report to Parks Australia: February 2002. John Woinarski, Michelle Watson and Nic Gambold Parks and Wildlife Commission of the Northern Territory PO Box 496 Palmerston Northern Territory, 0831. SUMMARY This report describes the results for the period January to December 2001 in the project Vertebrate Monitoring and Re-Sampling at Kakadu National Park (KNP), a collaborative project involving Parks Australia (North), Parks and Wildlife Commission of the Northern Territory, and the Tropical Savannas Cooperative Research Centre. The explicit objectives of this consultancy were to: • assess change in the vertebrate (and particularly mammal) fauna of KNP by re- sampling sites previously sampled; • assess the response of the mammal fauna to fire regimes, through sampling a set of sites selected to represent contrasting fire regimes; • establish a set of terrestrial vertebrate fauna samples which will contribute to the assessment of cane toad impacts; • sample terrestrial vertebrate fauna at a selection of existing KNP fire monitoring plots; • train Parks Australia staff in vertebrate sampling; and • collate all available data on terrestrial vertebrate fauna sampling, and deliver this to Parks Australia as GIS and other curated data bases. Note that this project will continue for a further year (2002). Hence, for some aspects of this project, the information presented here describes progress results rather than completed actions. monitoring and the Kakadu mammal fauna Prior to this work, there was no integrated monitoring program for terrestrial biodiversity across Kakadu NP. The development of such a program is important to assess the extent to which the Park’s values are being maintained and to help assess and guide management actions. -

Ecological Character Description of Toolibin Lake, Western Australia
ECOLOGICAL CHARACTER DESCRIPTION OF TOOLIBIN LAKE, WESTERN AUSTRALIA January 2006 Prepared for Department of Conservation and Land Management Prepared by Gary McMahon Ecosystem Solutions Pty Ltd PO Box 685 Dunsborough WA 6281 Ph: 08 9759 1960 Fax: 08 9759 1920 Limitations Statement This report has been exclusively drafted for the DEPARTMENT OF CONSERVATION & LAND MANAGEMENT. No express or implied warranties are made by Ecosystem Solutions Pty Ltd regarding the findings and data contained in this report. No new research or field studies were conducted. All of the information details included in this report are based upon the research provided and obtained at the time Ecosystem Solutions Pty Ltd conducted its analysis. In undertaking this work the authors have made every effort to ensure the accuracy of the information used. Any conclusions drawn or recommendations made in the report are done in good faith and the consultants take no responsibility for how this information and the report are used subsequently by others. Please note that the contents in this report may not be directly applicable towards another organisation’s needs or any other specific land area requiring management strategies. Ecosystem Solutions Pty Ltd accepts no liability whatsoever for a third party’s use of, or reliance upon, this specific report. Disclaimer The views and opinions expressed in this publication are those of the authors and do not necessarily reflect those of the Australian Government or the Minister for the Environment, or the Administrative Authority for Ramsar in Australia. While reasonable efforts have been made to ensure the contents of this publication are factually correct, the Commonwealth does not accept responsibility for the accuracy or completeness of the contents, and shall not be liable for any loss or damage that may be occasioned directly or indirectly through the use of, or reliance on, the contents of this publication.