For Perspectives in Plant Ecology, Evolution and Systematics Manuscript Draft
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Wildflowers to Grow in Your Garden Here Is the Key to the List Large
Wildflowers to grow in your garden Here is the key to the list Trees Ground covers Shrubs Eucalypts Banksias Myrtle family Banksias Others Baeckea Other Beaufortia Calothamnus Chamelaucium Hypocalymna Kunzea Melaleuca and Callistemon Scholtzia Thryptomene Verticordia Large trees. Think very carefully before you plant them! Large trees, such as lemon scented gums or spotted gums may look great in parks - at least local councils seem to think so (we would rather see local plants). But you may regret planting them in a modern small garden. That doesn't mean there is no room for trees. There are hundreds of attractive small trees that grow very well in native gardens. Here are just a few. Small trees Eucalypts with showy flowers. Eucalytpus caesia Comes in two sub species with the one known as "silver princess" being readily available in Perth. Lovely multi- stemmed weeping tree with pendulous pink flowers and silver-bell fruits. E. torquata Small upright tree with attractive pink flowers. Very drought resistant. E. ficifolia Often called the WA Flowering gum. Ranges in size from small to quite large and in flower colour from deep red to = Corymbia ficifolia orange to pale pink. In WA subject to a serious disease - called canker. Many trees succumb when about 10 or so years old, either dying or becoming very unhealthy. E. preissiana Bell fruited mallee. Small tree (or shrub) with bright yellow flowers. E. erythrocorys Illyarrie, red cap gum or helmet nut gum. Large golden flowers in February preceded by a bright red bud cap. Tree tends to be bit floppy and to need pruning. -

List of Plants Used by Carnaby's Black Cockatoo
Plants Used by Carnaby's Black Cockatoo List prepared by Christine Groom, Department of Environment and Conservation 15 April 2011 For more information on plant selection or references used to produce this list please visit the Plants for Carnaby's Search Tool webpage at www.dec.wa.gov.au/plantsforcarnabys Used for Soil type Soil drainage Priority for planting Sun Species Growth form Flower colour Origin for exposure Carnaby's Feeding Nesting Roosting Clayey Gravelly Loamy Sandy drained Well drained Poorly Waterlogged affected Salt Acacia baileyana (Cootamundra wattle)* Low Tree Yellow Australian native Acacia pentadenia (Karri Wattle) Low Tree Cream WA native Acacia saligna (Orange Wattle) Low Tree Yellow WA native Agonis flexuosa (Peppermint Tree) Low Tree White WA native Araucaria heterophylla (Norfolk Island Pine) Low Tree Green Exotic to Australia Banksia ashbyi (Ashby's Banksia) Medium Tree or Tall shrub Yellow, Orange WA native Banksia attenuata (Slender Banksia) High Tree Yellow WA native Banksia baxteri (Baxter's Banksia) Medium Tall shrub Yellow WA native Banksia carlinoides (Pink Dryandra) Medium Medium or small shrub White, cream, pink WA native Banksia coccinea (Scarlet Banksia) Medium Tree Red WA native Banksia dallanneyi (Couch Honeypot Dryandra) Low Medium or small shrub Orange, brown WA native Banksia ericifolia (Heath-leaved Banksia) Medium Tall shrub Orange Australian native Banksia fraseri (Dryandra) Medium Medium or small shrub Orange WA native Banksia gardneri (Prostrate Banksia) Low Medium -

PUBLISHER S Candolle Herbarium
Guide ERBARIUM H Candolle Herbarium Pamela Burns-Balogh ANDOLLE C Jardin Botanique, Geneva AIDC PUBLISHERP U R L 1 5H E R S S BRILLB RI LL Candolle Herbarium Jardin Botanique, Geneva Pamela Burns-Balogh Guide to the microform collection IDC number 800/2 M IDC1993 Compiler's Note The microfiche address, e.g. 120/13, refers to the fiche number and secondly to the individual photograph on each fiche arranged from left to right and from the top to the bottom row. Pamela Burns-Balogh Publisher's Note The microfiche publication of the Candolle Herbarium serves a dual purpose: the unique original plants are preserved for the future, and copies can be made available easily and cheaply for distribution to scholars and scientific institutes all over the world. The complete collection is available on 2842 microfiche (positive silver halide). The order number is 800/2. For prices of the complete collection or individual parts, please write to IDC Microform Publishers, P.O. Box 11205, 2301 EE Leiden, The Netherlands. THE DECANDOLLEPRODROMI HERBARIUM ALPHABETICAL INDEX Taxon Fiche Taxon Fiche Number Number -A- Acacia floribunda 421/2-3 Acacia glauca 424/14-15 Abatia sp. 213/18 Acacia guadalupensis 423/23 Abelia triflora 679/4 Acacia guianensis 422/5 Ablania guianensis 218/5 Acacia guilandinae 424/4 Abronia arenaria 2215/6-7 Acacia gummifera 421/15 Abroniamellifera 2215/5 Acacia haematomma 421/23 Abronia umbellata 221.5/3-4 Acacia haematoxylon 423/11 Abrotanella emarginata 1035/2 Acaciahastulata 418/5 Abrus precatorius 403/14 Acacia hebeclada 423/2-3 Acacia abietina 420/16 Acacia heterophylla 419/17-19 Acacia acanthocarpa 423/16-17 Acaciahispidissima 421/22 Acacia alata 418/3 Acacia hispidula 419/2 Acacia albida 422/17 Acacia horrida 422/18-20 Acacia amara 425/11 Acacia in....? 423/24 Acacia amoena 419/20 Acacia intertexta 421/9 Acacia anceps 419/5 Acacia julibross. -
Abelmoschus Moschatus Subsp
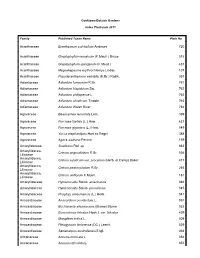
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Finschia-"A Genus of "Nut" Trees of the Southwest Pacific
Finschia-" A Genus of "Nut" Trees of the Southwest Pacific c. T. WHITE1 INTRODUCTION A PLANT FAMILY with a most interesting and F. Muell., Carnarvonia F. Muell., D arlin"gia F; intriguing distribution is Proteaceae, which finds Muell., Hollandaea F. Muell. (two spp.) , Mus its greatest development in Australia (650 " gravea F. Muell., and Placospermum White & species) on the one hand and South Africa (300 Francis. A surprising feature is the absence, species) on the other, though the two countries with the exception of one species in New Zea have no genera in common. Practically all the land, of the family "from Polynesia. South African species and the vast majority of There is in the islands of the southwest Paci "Australian ones are markedly xerophytic. The fic-Caroline Islands, New Guinea, Solomon largest genus, Greoillea R. Br., consists mainly Islands, and the New Hebrides-a group of trees of xerophytic shrubs or small trees but a few with the floral characters of Greuillea R. Br. are large trees found in the rain forests of and the fruit of Helicia Lour. These, I consider, tropical and subtropical eastern Australia, New all belong 'to Finschia Warb. This genus was Guinea, and New Caledonia. In the southwest founded by Warburg (1891: 297 ) on"a tree Pacific area the family finds its greatest develop from northeastern New Guinea. His original ment in northeastern Australia, where trees be description would cover Grevillea R. Br. exactly longing to it provide the great bulk of cabinet though he does not mention this genus and on timbers known in the trade as "Silky Oaks." the following page the distinctions he gives for There is close affinity between the Proteaceae of separating his proposed new genus from H elicia eastern Australia and of western South America are exactly those which distinguish Greuillea as illustrated by the genera Embothrium Forst. -

Australian Native Plants Society Australia Hakea
AUSTRALIAN NATIVE PLANTS SOCIETY AUSTRALIA HAKEA STUDY GROUP NEWSLETTER No. 65 OCTOBER 2017 ISSN0727- 7008 Leader: Paul Kennedy 210 Aireys Street Elliminyt Vic. 3250 E mail [email protected] Tel. 03-52315569 Dear members. We have had a very cold winter and now as spring emerges the cold remains with very wet conditions. Oh how I long for some warm sunshine to brighten our day. However the Hakeas have stood up to the cold weather very well and many have now flowered. Rainfall in August was 30mm but in the first 6 days of September another 56mm was recorded making the soil very moist indeed. The rain kept falling in September with 150mm recorded. Fortunately my drainage work of spoon drains and deeper drains with slotted pipe with blue metal cover on top shed a lot of water straight into the Council drains. Most of the Hakeas like well drained conditions, so building up beds and getting rid of excess water will help in making them survive. The collection here now stands at 162 species out of a possible 169. Seed of some of the remaining species hopefully will arrive here before Christmas so that I can propagate them over summer. Wanderings. Barbara and I spent most of June and July in northern NSW and Queensland to escape the cold conditions down here. I did look around for Hakeas and visited some members’ gardens. Just to the east of Cann River I found Hakea decurrens ssp. physocarpa, Hakea ulicina and Hakea teretifolia ssp. hirsuta all growing on the edge of a swamp. -

Newsletter No.100
AssociationAustralian of NativeSocieties Plants for Growing Society (Australia)Australian IncPlants Ref No. ISSN 0725-8755 Newsletter No. 100 – February 2015 GSG Vic Programme 2015 GSG SE Qld Programme 2015 Leader: Neil Marriott Meetings are usually held on the last Sunday 693 Panrock Reservoir Rd, Stawell, Vic. 3380 of the even months. We meet for a communal p 03 5356 2404 or 0458 177 989 morning tea at 9.30am after which the meetings e [email protected] commence at 10.00am. Visitors are always welcome. For more information or to check venues Contact Neil for queries about program for the etc please contact Bryson Easton on 0402 242 180 year. Any members who would like to visit the or Noreen Baxter on (07) 3871 3932 as changes official collection, obtain cutting material or seed, can occur. assist in its maintenance, and stay in our cottage for a few days are invited to contact Neil. Sunday, 22 February Venue: Home of Gail and Adrian Wockner, 5 Horizon Drive, Highfields Qld 4352 Time: 9:30am for 10am meeting Newsletter No. 100 No. Newsletter GSG NSW Programme 2015 Monday, 27 April For details contact Peter Olde 02 4659 6598. Venue: Mt Coot-tha Botanic Garden – meeting in the picnic sheds where road becomes two way 9:30am for 10am meeting Special thanks to the Victorian and New South Wales Time: chapters for this edition of the newsletter. Queensland Topic: Review of the Grevillea Gardens members, please note deadlines on back page for Note the change to Monday is so that members the following newsletter. -

Inventory of Taxa for the Fitzgerald River National Park
Flora Survey of the Coastal Catchments and Ranges of the Fitzgerald River National Park 2013 Damien Rathbone Department of Environment and Conservation, South Coast Region, 120 Albany Hwy, Albany, 6330. USE OF THIS REPORT Information used in this report may be copied or reproduced for study, research or educational purposed, subject to inclusion of acknowledgement of the source. DISCLAIMER The author has made every effort to ensure the accuracy of the information used. However, the author and participating bodies take no responsibiliy for how this informrion is used subsequently by other and accepts no liability for a third parties use or reliance upon this report. CITATION Rathbone, DA. (2013) Flora Survey of the Coastal Catchments and Ranges of the Fitzgerald River National Park. Unpublished report. Department of Environment and Conservation, Western Australia. ACKNOWLEDGEMENTS The author would like to thank many people that provided valable assistance and input into the project. Sarah Barrett, Anita Barnett, Karen Rusten, Deon Utber, Sarah Comer, Charlotte Mueller, Jason Peters, Roger Cunningham, Chris Rathbone, Carol Ebbett and Janet Newell provided assisstance with fieldwork. Carol Wilkins, Rachel Meissner, Juliet Wege, Barbara Rye, Mike Hislop, Cate Tauss, Rob Davis, Greg Keighery, Nathan McQuoid and Marco Rossetto assissted with plant identification. Coralie Hortin, Karin Baker and many other members of the Albany Wildflower society helped with vouchering of plant specimens. 2 Contents Abstract .............................................................................................................................. -

Table of Contents Below) with Family Name Provided
1 Australian Plants Society Plant Table Profiles – Sutherland Group (updated August 2021) Below is a progressive list of all cultivated plants from members’ gardens and Joseph Banks Native Plants Reserve that have made an appearance on the Plant Table at Sutherland Group meetings. Links to websites are provided for the plants so that further research can be done. Plants are grouped in the categories of: Trees and large shrubs (woody plants generally taller than 4 m) Medium to small shrubs (woody plants from 0.1 to 4 m) Ground covers or ground-dwelling (Grasses, orchids, herbaceous and soft-wooded plants, ferns etc), as well as epiphytes (eg: Platycerium) Vines and scramblers Plants are in alphabetical order by botanic names within plants categories (see table of contents below) with family name provided. Common names are included where there is a known common name for the plant: Table of Contents Trees and Large shrubs........................................................................................................................... 2 Medium to small shrubs ...................................................................................................................... 23 Groundcovers and other ground‐dwelling plants as well as epiphytes. ............................................ 64 Vines and Scramblers ........................................................................................................................... 86 Sutherland Group http://sutherland.austplants.com.au 2 Trees and Large shrubs Acacia decurrens -

7008 Australian Native Plants Society Australia Hakea
FEBRUARY 20 10 ISSN0727 - 7008 AUSTRALIAN NATIVE PLANTS SOCIETY AUSTRALIA HAKEA STUDY GROUP NEWSLETTER NUMBER 42 Leader: Paul Kennedy PO Box 220 Strathmerton,Vic. 3 64 1 e mail: hakeaholic@,mpt.net.au Dear members. The last week of February is drawing to a close here at Strathrnerton and for once the summer season has been wetter and not so hot. We have had one very hot spell where the temperature reached the low forties in January but otherwise the maximum daily temperature has been around 35 degrees C. The good news is that we had 25mm of rain on new years day and a further 60mm early in February which has transformed the dry native grasses into a sea of green. The native plants have responded to the moisture by shedding that appearance of drooping lack lustre leaves to one of bright shiny leaves and even new growth in some cases. Many inland parts of Queensland and NSW have received flooding rains and hopefully this is the signal that the long drought is finally coming to an end. To see the Darling River in flood and the billabongs full of water will enable regeneration of plants, and enable birds and fish to multiply. Unfortunately the upper reaches of the Murray and Murrurnbidgee river systems have missed out on these flooding rains. Cliff Wallis from Merimbula has sent me an updated report on the progress of his Hakea collection and was complaining about the dry conditions. Recently they had about 250mm over a couple of days, so I hope the species from dryer areas are not sitting in waterlogged soil. -

In This Issue in This Issue
No. 14 Hakea IN THIS ISSUE DHakea The first collection of This issue of Seed Notes Hakea was made in 1770 will cover the genus by Joseph Banks and Daniel Hakea. Solander from the Endeavour D Description expedition. The genus was described in 1797 by Schrader D Geographic and Wendland, and named distribution and habitat after Baron von Hake, a 19th century patron of botany, D Reproductive biology in Hanover. Plants were D Seed collection introduced into cultivation in England before that time. D Seed quality D assessment Hakea neurophylla. Photo – Sue Patrick D Seed germination D Recommended reading Description DMost hakeas are shrubs, woody and persistent; whereas ranging from small to low Grevillea has non-woody and medium height. They can non-persistent fruits. Most be useful for screening or as Hakea species have tough, groundcovers. Without fruits, pungent foliage that may be Hakea and Grevillea can be terete (needle-like), flat or confused. Both have flowers divided into segments. The with four tepals (petals and leaves are generally a similar sepals combined), an erect colour on both sides. Plants or recurved limb in bud and are usually single or multi- a similar range of leaf and stemmed shrubs, with smooth pollen presenter shapes. But bark, although there are the fruits are very different. ‘corkwood‘ hakeas with thick, Hakea fruits are generally deeply furrowed bark. Many Hakea can resprout after fire or disturbance, and these tend to be the species exhibiting multiple stems. The flowers are generally bisexual and range in colour from cream to green to pink, red, orange and mauve. -

Grow Natives in Pots
ATG About the Garden ATGFACTAbout SHEET the Garden Fact Sheet No. 21 GROWING NATIVES IN POTS The key factors to successfully growing Australian plants in containers are exactly the same as for exotic plants - drainage and nutrition. The main points to remember are that good drainage is even more important for certain natives (mainly those originating in arid areas with very light, sandy soils) and that plants in the Proteaceae family require low phosphorus fertilisers. When growing plants in containers it is important *PLANTS IN THE to know their type and origin so ask yourself the following questions: FAMILY PROTEACEAE • Tree, shrub or climber/groundcover? • Does it originate in a high or low rainfall area? INCLUDE: • Is its natural habitat sun or shade? • Natural soil type - light (sandy) or heavy (clay)? Athertonia banksia The type of plant determines the ultimate pot size. buckinghamia Knowing its origins will tell you the type of growing cardwellia medium you need, how much water and whether it darlingia should be placed in sun or shade. If the label doesn’t dryandra give you enough information, ask nursery staff. hakea lomatia Any type of container can be used, providing it has macadamia plenty of adequate sized drainage holes. Too small and oreocallis they will drain too slowly and block up easily; too large persoonia and all your precious water will run away too fast. Most petrophile, good quality terracotta and plastic pots come with stenocarpus and good drainage holes; a medium-sized pot should have telopea no less than four, about the size of a five cent piece.