Polyphenol Content and Antioxidant Activity of Stevia and Peppermint As a Result of Organic and Conventional Fertilization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Optimization of Microwave Assisted Process for Extraction of Celery Seed Essential Oil Gopika Talwari1 and B.S
Gopika Talwari and B.S. Ghuman JAE : 51 (2) Journal of Agricultural Engineering Vol. 51 (2): April-June, 2014 Optimization of Microwave Assisted Process for Extraction of Celery Seed Essential Oil Gopika Talwari1 and B.S. Ghuman2 Manuscript received: March, 2013 Revised manuscript accepted: April, 2014 ABSTRACT Microwave assisted extraction (MAE) method was developed for extraction of essential oil from celery seeds. A domestic microwave oven was modified and Clevenger apparatus attached to it to make it an extraction unit. Effect of various parameters such as soaking time, temperature and power density during MAE was studied. A multivariate study based on a Box-Behnken design was used to evaluate the influence of three major variables (soaking time, temperature and power density) affecting the performance of MAE on celery seed. Oil yield, time of extraction and energy consumption (MJ.kg-1 oil) by MAE were determined and compared with those obtained by the traditional hydro-distillation (HD). It was found that microwave assisted process gave approximately same oil yield (1.90%) in less time ( 93.5 min) and with low energy consumption (58191.78 MJ.kg-1 oil). Results revealed that the selected parameters had significant effect on the responses. Key words: Celery seed, essential oil, microwave assisted extraction, hydro distillation Essential oils are the volatile oils distilled from aromatic an average contains 2.5% volatile oil containing 60-70% plant material. Essential oils are contained in the glands, d-limonene and 1-20% beta selinene and 15%–17% fixed sacs and veins concentrated in different parts of the plant. oil. -

Volatiles of Black Pepper Fruits (Piper Nigrum L.)
molecules Article Volatiles of Black Pepper Fruits (Piper nigrum L.) Noura S. Dosoky 1 , Prabodh Satyal 1, Luccas M. Barata 2 , Joyce Kelly R. da Silva 2 and William N. Setzer 1,3,* 1 Aromatic Plant Research Center, Suite 100, Lehi, UT 84043, USA; [email protected] (N.S.D.); [email protected] (P.S.) 2 Programa de Pós-Graduação em Biotecnologia, Universidade Federal do Pará, Belém 66075-110, PA, Brazil; [email protected] (L.M.B.); [email protected] (J.K.R.d.S.) 3 Department of Chemistry, University of Alabama in Huntsville, Huntsville, AL 35899, USA * Correspondence: [email protected]; Tel.: +1-256-824-6519 Academic Editor: Francesca Mancianti Received: 4 October 2019; Accepted: 5 November 2019; Published: 21 November 2019 Abstract: Black pepper (Piper nigrum) is historically one of the most important spices and herbal medicines, and is now cultivated in tropical regions worldwide. The essential oil of black pepper fruits has shown a myriad of biological activities and is a commercially important commodity. In this work, five black pepper essential oils from eastern coastal region of Madagascar and six black pepper essential oils from the Amazon region of Brazil were obtained by hydrodistillation and analyzed by gas chromatography-mass spectrometry. The major components of the essential oils were α-pinene, sabinene, β-pinene, δ-3-carene, limonene, and β-caryophyllene. A comparison of the Madagascar and Brazilian essential oils with black pepper essential oils from various geographical regions reported in the literature was carried out. A hierarchical cluster analysis using the data obtained in this study and those reported in the literature revealed four clearly defined clusters based on the relative concentrations of the major components. -

Physiological Responses of Ocimum Basilicum, Salvia Officinalis, And
plants Article Physiological Responses of Ocimum basilicum, Salvia officinalis, and Mentha piperita to Leaf Wounding Konstantinos Vrakas, Efterpi Florou, Athanasios Koulopoulos and George Zervoudakis * Department of Agriculture, University of Patras, Terma Theodoropoulou, 27200 Amaliada, Greece; [email protected] (K.V.); evtefl[email protected] (E.F.); [email protected] (A.K.) * Correspondence: [email protected] Abstract: The investigation about the leaf wounding effect on plant physiological procedures and on leaf pigments content will contribute to the understanding of the plants’ responses against this abiotic stress. During the experiment, some physiological parameters such as photosynthesis, transpiration and stomatal conductance as well as the chlorophyll and anthocyanin leaf contents of Ocimum basilicum, Salvia officinalis, and Mentha piperita plants were measured for about 20–40 days. All the measurements were conducted on control and wounded plants while in the latter, they were conducted on both wounded and intact leaves. A wide range of responses was observed in the wounded leaves, that is: (a) immediate decrease of the gas exchange parameters and long-term decrease of almost all the measured variables from O. basilicum, (b) immediate but only short-term decrease of the gas exchange parameters and no effect on pigments from M. piperita, and (c) no effect on the gas exchange parameters and decrease of the pigments content from S. officinalis. Regarding the intact leaves, in general, they exhibited a similar profile with the control ones for all plants. These results imply that the plant response to wounding is a complex phenomenon depending on plant species and the severity of the injury. Citation: Vrakas, K.; Florou, E.; Koulopoulos, A.; Zervoudakis, G. -

Nutritional and Medicinal Properties of Stevia Rebaudiana
Review Article Curr Res Diabetes Obes J Volume 13 Issue 4 - July 2020 Copyright © All rights are reserved by Fasiha Ahsan DOI: 10.19080/CRDOJ.2020.13.555867 Nutritional and Medicinal Properties of Stevia Rebaudiana Fasiha Ahsan*, Shahid Bashir and Faiz-ul-Hassan Shah University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Submission: June 25, 2020; Published: July 16, 2020 *Corresponding author: Fasiha Ahsan, PhD Scholar, University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Abstract Researches on new molecules with the least toxic effects and better potency is on its way and more attention is being given upon medicinal plants for forcing away the above problems. Medicinal plants have been recognized as potential drug candidates. Stevia, a natural sweetener with medicinal properties and also having nutritional, therapeutic and industrial importance is being used all over the world. Stevia rebaudiana leaves are usually referred to as candy, sweet and honey leaves. Diterpene glycosides are responsible for its high sweetening potential of leaves. The phytochemical properties of bioactive chemicals present in stevia leaves are involves in maintaining the physiological functions of human body. Paper also highlights the importance of nutritional aspects of dried stevia leaves, metabolism of stevia, effects of it consumption on human health and clinical studies related to stevia ingestion. Various medicinal properties of stevia leaves discussed in paper like anti-hyperglycemia, anti-oxidative, hypotensive, nephro-protective, hepato protective, antibacterial and antifungal. Basic purpose of this review to understand the medicinalKeywords: potential Stevia; Diabetes;of stevia and Phytochemicals; its acceptance Medicinal as a significant plant; Steviol;raw material Nutrition; for human Disorders diet. -
Show Activity
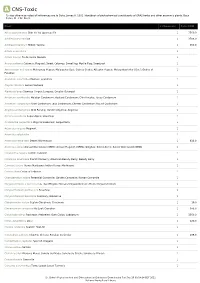
A CNS-Toxic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Achillea moschata Iva 1 3708.0 Achillea millefolium Milfoil; Yarrow 1 550.0 Acinos suaveolens 1 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Calamus; Flagroot; Sweet Calamus; Sweetflag; Myrtle Flag; Sweetroot 1 Aframomum melegueta Melegueta Pepper; Malagueta (Sp.); Guinea Grains; Alligator Pepper; Malagettapfeffer (Ger.); Grains-of- 1 Paradise Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 Amomum xanthioides Malabar Cardamom; Bastard Cardamom; Chin Kousha; Tavoy Cardamom 1 Amomum compactum Siam Cardamom; Java Cardamom; Chester Cardamom; Round Cardamom 1 Angelica archangelica Wild Parsnip; Garden Angelica; Angelica 1 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Virginia Snakeroot; Serpentaria 1 Artemisia vulgaris Mugwort 1 Artemisia salsoloides 1 Artemisia herba-alba Desert Wormwood 1 638.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Qinghao; Sweet Annie; Sweet Wormwood (GRIN) 1 Calamintha nepeta Turkish Calamint 1 Callicarpa americana French Mulberry; American Beauty Berry; Beauty Berry 1 Cannabis sativa Hemp; Marijuana; Indian Hemp; Marihuana 1 Cedrus libani Cedar of Lebanon 1 Chamaemelum nobile Perennial Camomile; Garden Camomile; Roman Camomile -

How Do Mentha Plants Induce Resistance Against Tetranychus Urticae (Acari: Tetranychidae) in Organic Farming?
Journal of Plant Protection Research ISSN 1427-4345 ORIGINAL ARTICLE How do mentha plants induce resistance against Tetranychus urticae (Acari: Tetranychidae) in organic farming? Sally Farouk Allam1, Basem Abdel-Nasser Soudy2*, Ahmed Salah Hassan1, Mahmoud Mohamed Ramadan3, Doha Abo Baker4 1 Zoology and Agricultural Nematology Department, Faculty of Agriculture, Cairo University, Giza, Egypt 2 Applied Centre of Entomonematodes, Faculty of Agriculture, Cairo University, Giza, Egypt 3 Pests and Plant Protection Department, National Research Centre, Dokki, Giza, Egypt 4 Medicinal and Aromatic Plants Department, Pharmaceutical and Drug Discovery Division, National Research Centre, Dokki, Giza, Egypt Vol. 58, No. 3: 265–275, 2018 Abstract DOI: 10.24425/122943 Tetranychus urticae (Acari: Tetranychidae) infesting many plants but Mentha viridis L., and Mentha piperita L., were low in number of infestation. Therefore the objective of this study Received: March 13, 2018 was to identify the resistance of M. viridis and M. piperita plants against T. urticae by study- Accepted: July 24, 2018 ing the external shape and internal contents of those plants. For morphological studies, dried leaves were covered with gold utilizing an Edwards Scan coat six sputter-coater. For *Corresponding address: histological studies, arrangements of Soft Tissue technique were used. For phytochemi- [email protected] cal studies, the plants were cut, dried and then high performance liquid chromatography (HPLC) was used. While feeding the mites were collected from the area between oily glands, trichomes and respiratory stomata in both mint species. The most important leaf structures in aromatic plants are the oily glands found on the external part of the leaves (both upper and lower epidermis). -

Sharp's at Waterford Farm Your Neighborhood Farm Ask Us How To
Lemongrass – Essential for Thai Sharp’s at Waterford Herbs List cooking Farm Anise - Hyssop Lovage (Levistcum officinale) Farming in Howard County Basil Marjoram (Origanum majorana) since 1903 African Blue Amethyst Improved Purple Sweet Eleonora Zaatar, a hint of thyme, oregano & 4003 Jennings Chapel Rd. Elidia - Compact; container basil marjoram Brookeville, MD 20833 Genovese Golden - ornamental mostly Holy - Sacred Red and Green Tel: (410) 489-2572 Mint (Mentha sp.) Italian Large Leaf Chocolate Peppermint Lemon – Mrs. Burns www.sharpfarm.com Lemon Mint Mountain Mint Lettuce Leaf – Napoletano email: Peppermint Pineapple Mint Lime [email protected] Spearmint Sweet Thai Dark Opal Oregano (Origanum sp.) Red Rubin Greek Rutgers Devotion Zaatar ( a hint of thyme, oregano, & marjoram) Oreganum Syriaca) Borage: the herb of gladness Hot and Spicy - real tang, our favorite for adding to beans Catnip (Nepeta)- feline friends treat Parsley (Petroselinum crispum) Calendula, Neon Plain leaf (Italian or flat) Curly – double or triple Chamomile (German) Organic curled parsley (Bodegold) Italian Dark Green – Giant of Italy – huge leaves Your Neighborhood Chervil (Anthricus cerefolium) ‘crispum’ Vertissimo Farm Rosemary (Rosmarinus) Arp Chives (Allium) Hill Hardy Med Leaf (Purly) Ask Us How to Garden Salem Large leaf (staro) Sage (Salvia offincinalis) Helpful Hints: We pride ourselves Cilantro (Coriandrum sativium) Garden - Extrakta on knowing how to vegetable and herb Cruiser – more upright – great for Pineapple garden. Please ask if you need bunching – 50 days Savory Winter information on how to. Yields? Cutting Celery (Apium graveolens) Sorrel, French Spacing between plants? Staking? aka leaf celery When you plant, space your harvest Stevia (Stevia rebaudiana) by using varieties of different maturity Dill (Anethum graveolens): Nature’s natural sweetener dates. -

Steviol Glycosides from Stevia Rebaudiana Bertoni
0 out of 21 Residue Monograph prepared by the meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017 Steviol Glycosides from Stevia rebaudiana Bertoni This monograph was also published in: Compendium of Food Additive Specifications. Joint FAO/WHO Expert Committee on Food Additives (JECFA), 84th meeting 2017. FAO JECFA Monographs 20 © FAO/WHO 2017 1 out of 21 STEVIOL GLYCOSIDES FROM STEVIA REBAUDIANA BERTONI Prepared at the 84th JECFA (2017) and published in FAO JECFA Monographs 20 (2017), superseding tentative specifications prepared at the 82nd JECFA (2016) and published in FAO JECFA Monographs 19 (2016). An ADI of 0 - 4 mg/kg bw (expressed as steviol) was established at the 69th JECFA (2008). SYNONYMS INS No. 960 DEFINITION Steviol glycosides consist of a mixture of compounds containing a steviol backbone conjugated to any number or combination of the principal sugar moieties (glucose, rhamnose, xylose, fructose, arabinose, galactose and deoxyglucose) in any of the orientations occurring in the leaves of Stevia rebaudiana Bertoni. The product is obtained from the leaves of Stevia rebaudiana Bertoni. The leaves are extracted with hot water and the aqueous extract is passed through an adsorption resin to trap and concentrate the component steviol glycosides. The resin is washed with a solvent alcohol to release the glycosides and the product is recrystallized from methanol or aqueous ethanol. Ion exchange resins may be used in the purification process. The final product may be spray-dried. Chemical name See Appendix 1 C.A.S. number See Appendix 1 Chemical formula See Appendix 1 Structural formula Steviol (R1 = R2 = H) is the aglycone of the steviol glycosides. -

Selection of Suitable Propagation Method for Consistent Plantlets Production in Stevia Rebaudiana (Bertoni)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Saudi Journal of Biological Sciences (2014) 21, 566–573 King Saud University Saudi Journal of Biological Sciences www.ksu.edu.sa www.sciencedirect.com ORIGINAL ARTICLE Selection of suitable propagation method for consistent plantlets production in Stevia rebaudiana (Bertoni) Shahid Akbar Khalil a,*, Roshan Zamir a, Nisar Ahmad b a Nuclear Institute for Food and Agriculture (NIFA), Peshawar, Pakistan b Department of Biotechnology, Faculty of Biological Sciences, Quaid-i-Azam University, Islamabad 45320, Pakistan Received 11 December 2013; revised 10 February 2014; accepted 20 February 2014 Available online 5 March 2014 KEYWORDS Abstract Stevia rebaudiana (Bert.) is an emerging sugar alternative and anti-diabetic plant in Paki- Stevia rebaudiana; stan. That is why people did not know the exact time of propagation. The main objective of the Seed germination; present study was to establish feasible propagation methods for healthy biomass production. In Seed radiation; the present study, seed germination, stem cuttings and micropropagation were investigated for Stem cuttings; higher productivity. Fresh seeds showed better germination (25.51–40%) but lost viability after a Micropropagation few days of storage. In order to improve the germination percentage, seeds were irradiated with 2.5, 5.0, 7.5 and 10 Gy gamma doses. But gamma irradiation did not show any significant change in seed germination. A great variation in survival of stem cutting was observed in each month of 2012. October and November were found the most suitable months for stem cutting survival (60%). -

Impact of Spirulina Platensis and Stevia Rebaudiana on Growth and Essential Oil Production of Basil Ocimum Citriodorum
IMPACT OF SPIRULINA PLATENSIS AND STEVIA REBAUDIANA ON GROWTH AND ESSENTIAL OIL PRODUCTION OF BASIL OCIMUM CITRIODORUM Sherif S. Saleh and Nahed S.A. El-Shayeb To cite the article: Sherif S. Saleh and Nahed S.A. El-Shayeb (2020), Impact of Spirulina platensis and Stevia rebaudiana on growth and essential oil production of basil Ocimum citriodorum, Journal of Agricultural and Rural Research, 3(2): 78-93. Link to this article: http://aiipub.com/journals/jarr-200329-010098/ Article QR Journal QR JOURNAL OF AGRICULTURAL AND RURAL RESEARCH VOL. 4, ISSUE 2, PP. 78-93. http://aiipub.com/journal-of-agricultural-and-rural-research-jarr/ IMPACT OF SPIRULINA PLATENSIS AND STEVIA REBAUDIANA ON GROWTH AND ESSENTIAL OIL PRODUCTION OF BASIL OCIMUM CITRIODORUM Sherif S. Saleh 1,2* and Nahed S.A. El-Shayeb2 1 Tissue Culture and Nanotechnology Lab., Hort., Res., Institute, A. R. C., Egypt 2 Medicinal and Aromatic Department, Hort., Res., Institute, A. R. C., Egypt A R T I C L E I N F O A B S T R A C T Article Type: Research The constituents of essential oils isolated by diethyl ether of the in Received: 20, Mar. 2020. vitro explants of Ocimum citriodorum cultured on alternative media Accepted: 04, Apr. 2020. containing green algae spirulina and stevia plant powder or filtrate Published: 04, Apr. 2020. were examined by GC-MS. The Spirulina platensis green algae were added in culture medium powder at (0.5, 1.0 and 2.0 g/l) and powder Keywords: filtrated at 2, 4 and 6 ml/l from concentrations 5, 10 and 15 %, Ocimum citriodorum, stevia respectively. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

Effect of Different Drying Techniques on the Volatile Compounds, Morphological Characteristics and Thermal Stability of Stevia Rebaudiana Bertoni Leaf
Gasmalla et al Tropical Journal of Pharmaceutical Research June 2017; 16 (6): 1399-1406 ISSN: 1596-5996 (print); 1596-9827 (electronic) © Pharmacotherapy Group, Faculty of Pharmacy, University of Benin, Benin City, 300001 Nigeria. All rights reserved. Available online at http://www.tjpr.org http://dx.doi.org/10.4314/tjpr.v16i6.26 Original Research Article Effect of different drying techniques on the volatile compounds, morphological characteristics and thermal stability of Stevia rebaudiana Bertoni leaf Mohammed Abdalbasit A Gasmalla1,2, Habtamu A Tessema3, Kamal Alahmed3, Xiao Hua3, Xiangru Liao4 and RuijinYang1,3* 1State Key Laboratory of Food Science & Technology, Jiangnan University, 1800 Lihu Avenue, Wuxi 214122, Jiangsu, China, 2Department of Nutrition & Food technology, Faculty of Science and Technology, Omdurman Islamic University, PO Box 382, 14415, Khartoum, Sudan, 3School of Food Science and Technology, Jiangnan University, 1800 Lihu Avenue, 4The Key Laboratory of Industrial Biotechnology, Ministry of Education, School of Biotechnology, Jiangnan University, 1800 Lihu Road, Wuxi 214122, Jiangsu, China *For correspondence: Email: [email protected]; Tel: + 86-510-85919150; Fax: +86510-85919150 Sent for review: 3 December 2016 Revised accepted: 13 May 2017 Abstract Purpose: To examine the volatile compounds, thermal stability and morphological characteristics of stevia (Stevia rebaudiana Bertoni) leaves after sun, oven and microwave drying. Methods: Gas chromatography-mass spectrometry with a spectral analysis manager was used to separate the volatile compounds. Dried stevia leaf powder was characterized morphologically by scanning electron microscopy while thermal properties were determined by differential scanning calorimetry (DSC). Results: The plant material contained large amounts of spathulenol and caryophyllene oxide. The main compounds were 1-docosanol and hexanoic acid; trans-β-ionone, 5-methylundecane, 2,5,6- trimethyldecane, (+) spathulenol, propanoic acid and 1-chlorononadecane.