Research Journal of Pharmaceutical, Biological and Chemical Sciences
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ruta Graveolens L. Essential Oil Composition Under Different Nutritional Treatments
American-Eurasian J. Agric. & Environ. Sci., 13 (10): 1390-1395, 2013 ISSN 1818-6769 © IDOSI Publications, 2013 DOI: 10.5829/idosi.aejaes.2013.13.10.11248 Ruta graveolens L. Essential Oil Composition under Different Nutritional Treatments 12Afaq Ahmad Malik, Showkat R. Mir and 1Javed Ahmad 1Department of Botany, Jamia Hamdard, New Delhi 110062, India 2Department of Pharmacognosy and Phytochemistry, Jamia Hamdard, New Delhi 110 062, India Abstract: The use of un-exploited organic industrial by-products and municipal wastes as soil organic amendment has an economic value and environmental interest. However, little is known about their effectiveness on medicinal plants cultivation. An experiment was conducted in this regard to assess the impact of farmyard manure (FYM), composted sugarcane pressmud (CPM) and sewage sludge biosolid (SSB) on volatile oil composition of Ruta graveolens L., an important aromatic medicinal herb used frequently in Unani system of medicine in India. Volatile oil in the aerial parts of the plant was isolated by hydro-distillation and analyzed by GC-MS. Hydro-distillation of untreated (control) plants yielded 0.32% essential oil on fresh weight basis. The predominant components in the essential oil were n-Hex-4-en-3-one (55.06%), n-Pent-3-one (28.17%), n-Hex-3-en-2-one (14.07%) and n-Hex-5-en-3-one (0.67%). Essential oil obtained from plants treated with FYM amounted to 0.36% of fresh weight and consisted mainly of n-Hex-4-en-3-one (53.64%), n-Pent-3-one (37.82%) and n-Hex-3-en-2-one (7.22%). -

Effects of the Daily Consumption of Stevia on Glucose
nutrients Article Effects of the Daily Consumption of Stevia on Glucose Homeostasis, Body Weight, and Energy Intake: A Randomised Open-Label 12-Week Trial in Healthy Adults Nikoleta S. Stamataki 1 , Benjamin Crooks 1,2 , Abubaker Ahmed 1,3 and John T. McLaughlin 1,2,* 1 Division of Diabetes, Endocrinology & Gastroenterology, School of Medical Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Sciences Centre, The University of Manchester, Oxford Rd, Manchester M13 9PL, UK; [email protected] (N.S.S.); [email protected] (B.C.); [email protected] (A.A.) 2 Department of Gastroenterology, Salford Royal NHS Foundation Trust, Stott Lane, Salford M6 8HD, UK 3 Department of Gastroenterology, Manchester Foundation Trust, Oxford Road, Manchester M13 9WL, UK * Correspondence: [email protected] Received: 3 September 2020; Accepted: 2 October 2020; Published: 6 October 2020 Abstract: Stevia is a non-nutritive sweetener, providing sweet taste with no calories. This randomised, controlled, open-label 2-parallel arm trial examined the effects of daily stevia consumption on glycaemia in healthy adults. Secondary endpoints included body weight (BW) and energy intake (EI). Healthy participants (n = 28; aged 25 5y, body mass index 21.2 1.7 kg/m2) were randomised ± ± into either the stevia group (n = 14)—required to consume a stevia extract daily—or to the control group (n = 14). At weeks 0 and 12, the glucose and insulin responses to an oral glucose tolerance test were measured; BW and EI were assessed at weeks 0, 6, and 12. There was no significant difference in the glucose or insulin responses. -

Tips to Roast Vegetables Spice Guide
Tips to Roast Vegetables • Roast at a high oven temp- 400 to 450 degrees F • Chop vegetables in uniform size so they cook evenly • Don’t over crowd the pan, otherwise they will become soft • Roasting veggies with some oil will help them become crispier • To get the most flavor/crispier roast them on the top rack • Seasoning before putting them in the oven will add flavor • Flip veggies halfway through to ensure even cooking • When roasting multiple types of veggies, ensure they have similar cooking times. Good pairs include: Cauliflower and Broccoli cc Carrots and Broccoli Baby potatoes and Butternut Squash Onions and Bell Peppers Zucchini and Yellow Squash Asparagus and Leeks Spice Guide Table of Contents Spices by Cuisine Herbs and Spices 1 Mexican Coriander, Cumin, oregano, garlic powder, cinnamon, chili powder Herbs and Spices that Pair well with Proteins 2 Caribbean Chicken Fajita Bowl Recipe 3 All spice, nutmeg, garlic powder, cloves, cinnamon, ginger Shelf life of Herbs and Spices 4 French Nutmeg, thyme, garlic powder, rosemary, oregano, Herbs de Provence Spices by Cuisine 5 North African Tips to Roast Vegetables BP Cardamum, cinnamon, cumin, paprika, turmeric, ginger Cajun Cayenne, oregano, paprika, thyme, rosemary, bay leaves, Cajun seasoning Thai Basil, cumin, garlic, ginger, turmeric, cardamum, curry powder Mediterranean Oregano, rosemary, thyme, bay leaves, cardamum, cinnamon, cloves, coriander, basil, ginger Indian Bay leaves, cardamum, cayenne, cinnamon, coriander, cumin, ginger, nutmeg, paprika, turmeric, garam masala, curry powder Middle Eastern Bay leaves, cardamum, cinnamon, cloves, cumin, ginger, coriander, oregano, za’atar, garlic powder 5 Shelf Life of Herbs and Herbs and Spices Spices Herbs Herbs are plants that’s leaves can be used to add flavor to foods. -

Entomotoxicity of Xylopia Aethiopica and Aframomum Melegueta In
Volume 8, Number 4, December .2015 ISSN 1995-6673 JJBS Pages 263 - 268 Jordan Journal of Biological Sciences EntomoToxicity of Xylopia aethiopica and Aframomum melegueta in Suppressing Oviposition and Adult Emergence of Callasobruchus maculatus (Fabricus) (Coleoptera: Chrysomelidae) Infesting Stored Cowpea Seeds Jacobs M. Adesina1,3,*, Adeolu R. Jose2, Yallapa Rajashaker3 and Lawrence A. 1 Afolabi 1Department of Crop, Soil and Pest Management Technology, Rufus Giwa Polytechnic, P. M. B. 1019, Owo, Ondo State. Nigeria; 2 Department of Science Laboratory Technology, Environmental Biology Unit, Rufus Giwa Polytechnic, P. M. B. 1019, Owo, Ondo State. Nigeria; 3 Insect Bioresource Laboratory, Institute of Bioresources and Sustainable Development, Department of Biotechnology, Government of India, Takyelpat, Imphal, 795001, Manipur, India. Received: June 13, 2015 Revised: July 3, 2015 Accepted: July 19, 2015 Abstract The cowpea beetle, Callosobruchus maculatus (Fabricus) (Coleoptera: Chrysomelidae), is a major pest of stored cowpea militating against food security in developing nations. The comparative study of Xylopia aethiopica and Aframomum melegueta powder in respect to their phytochemical and insecticidal properties against C. maculatus was carried out using a Complete Randomized Design (CRD) with five treatments (0, 1.0, 1.5, 2.0 and 2.5g/20g cowpea seeds corresponding to 0.0, 0.05, 0.075, 0.1 and 0.13% v/w) replicated thrice under ambient laboratory condition (28±2°C temperature and 75±5% relative humidity). The phytochemical screening showed the presence of flavonoids, saponins, tannins, cardiac glycoside in both plants, while alkaloids was present in A. melegueta and absent in X. aethiopica. The mortality of C. maculatus increased gradually with exposure time and dosage of the plant powders. -

Cilantro Dill Rosemary Ginger Mint Basil
Dill Rosemary Basil Herbs Ginger Cilantro Mint What is an Herb? • Plants that are used as flavoring agents • Leaves, seeds or roots can be used • Usually used in small amounts • Many may be used for medicinal or ornamental purposes Basil Basil • Mint-like annual herb used for cooking, garnish, or medicinal purposes • Readily cross pollinates and several hybrids available • Grown in plots of less than 0.1 acre for local sales • A source of organic insecticide and fungicide • Pests: Japanese beetle; annual weeds • Disease: Botrytis, leaf blight, Sclerotinia blight, Fusarium wilt Mint Mint • Perennial, grown from vegetative material • Multiple harvests from a field, sold fresh • Pests: Loopers and Cutworms • Diseases: Verticillium wilt and Rust • Produced by 15 to 25 commercial growers in Texas • Menthols and esters are distilled from peppermint and spearmint in the Pacific Northwest Cilantro – Soil Preparation • Prefers a light, well-drained, moderately fertile loam or sandy soil • Can tolerate other soil conditions Cilantro - Planting • Will start to bolt when temperatures exceed 85 degrees F • Plant in February for April harvest; September for November harvest • Plant seeds 2 inches apart in rows 12 to 15 inches apart if plan to harvest leaves • Plant seeds 8 inches apart in rows 15 inches apart if plan to harvest seeds Cilantro - Planting • Plant seeds about ¼ to ½ inch deep • About 2,000 seeds per ounce, so don’t purchase a lot of seeds for the season • Weekly planting will ensure continuous crop Cilantro - Fertilizing • Should be fertilized -

Never Before Seen Secret Recipes from GFB.Org
Never Before Seen Secret Recipes from GFB.org Welcome to Gluten-Free-Bread.org! On behalf of our team here, I’d like to personally welcome you to our community. Our goal, in everything we publish, is to be your go-to resource on the things that impact you the most; whether its dining out, educating the public, links between gluten and other conditions, or just recipes to bake to your hearts content. YOU are the drive behind this website and this community. Your reviews and feedback help us make this community a ‘happening’ place to be. So since YOU are so important to us, we want to get to know you. I have 2 requests that will help us get to know you and your needs… 1. If you haven’t connected with us yet on our social media communities, check us out on Facebook, Twitter, Pinterest and Google + 2. And, what specifically are you looking for – a particular recipe, a new ingredient, product reviews, tips, helpful hints – what would help you out the most? Reply to ANY email you receive from us at ANY time and let us know. ©Gluten-Free-Bread.org And now, for your Never Before Seen Secret Recipes! Enjoy, from our kitchen to yours… Cinnamon Roll Scones Scone Ingredients: 2 cups almond flour 2 tbsp granulated erythritol* 2 tsp baking powder 1/2 tsp baking soda 1/2 tsp salt 1/4 tsp ground cinnamon 1 large egg, lightly beaten 1/4 cup coconut oil, melted (you may use butter if you prefer) 2 tbsp heavy cream 1/2 tsp vanilla extract 8 drops stevia extract Filling/Topping: 3 tbsp granulated erythritol 2 tsp cinnamon Icing: 1 oz cream cheese, softened ©Gluten-Free-Bread.org 1 tbsp heavy cream 1/2 tbsp butter, softened 1 tbsp powdered erythritol 1/4 tsp vanilla extract 6 drops stevia extract For the scones, line a baking sheet with parchment paper and preheat oven to 325°F. -

Andrew's Rogan Josh Recipe Ingredients
Andrew’s Rogan Josh Recipe Ingredients: - Garlic/Ginger paste(4 garlic cloves and about 1 inch of fresh ginger chopped if you can’t get the paste - blended with water into a paste) - 2 Tablespoons of oil - 1 lb of meat chopped into cubes (lamb is best but you can use beef or chicken) - 2 or 3 tomatoes (chopped) - 2 onions (finely chopped) - 5 cardamom pods (whole) - 4 cloves - 1 or 2 bay leaves - 5 or 6 black pepper corns - 1 and 1/2 cinnamon sticks - Good teaspoon of cumin seeds - 1 teaspoon of ground coriander - 2 teaspoons of paprika - 1 teaspoon of cayenne pepper - 3 or 4 tablespoons of plain yogurt - 1/4 teaspoon of garam masala (not essential - I usually forget about it) - Juice of 1/2 a lemon Heat the oil and brown the meat cubes. Take the meat out and set it to one side. Put the bay leaves, cloves, cardamom, pepper corns and cinnamon into the oil while it’s still hot. Stir it around until the bay leaves change colour and the cloves begin to swell. Add the onions and fry for 5 minutes or so - until they turn a lovely brown colour. Add the ginger/garlic paste and stir for 30 seconds. Now add the coriander, cumin, cayenne and paprika and stir for another 30 seconds. Return the meat and its juices to the pan and stir for 30 seconds. Add the tomato and after a minute or so add 1 tablespoon of yogurt. After stirring in the yogurt until it’s well blended, add the rest of the yogurt. -

Physiological Responses of Ocimum Basilicum, Salvia Officinalis, And
plants Article Physiological Responses of Ocimum basilicum, Salvia officinalis, and Mentha piperita to Leaf Wounding Konstantinos Vrakas, Efterpi Florou, Athanasios Koulopoulos and George Zervoudakis * Department of Agriculture, University of Patras, Terma Theodoropoulou, 27200 Amaliada, Greece; [email protected] (K.V.); evtefl[email protected] (E.F.); [email protected] (A.K.) * Correspondence: [email protected] Abstract: The investigation about the leaf wounding effect on plant physiological procedures and on leaf pigments content will contribute to the understanding of the plants’ responses against this abiotic stress. During the experiment, some physiological parameters such as photosynthesis, transpiration and stomatal conductance as well as the chlorophyll and anthocyanin leaf contents of Ocimum basilicum, Salvia officinalis, and Mentha piperita plants were measured for about 20–40 days. All the measurements were conducted on control and wounded plants while in the latter, they were conducted on both wounded and intact leaves. A wide range of responses was observed in the wounded leaves, that is: (a) immediate decrease of the gas exchange parameters and long-term decrease of almost all the measured variables from O. basilicum, (b) immediate but only short-term decrease of the gas exchange parameters and no effect on pigments from M. piperita, and (c) no effect on the gas exchange parameters and decrease of the pigments content from S. officinalis. Regarding the intact leaves, in general, they exhibited a similar profile with the control ones for all plants. These results imply that the plant response to wounding is a complex phenomenon depending on plant species and the severity of the injury. Citation: Vrakas, K.; Florou, E.; Koulopoulos, A.; Zervoudakis, G. -

Companion Plants for Better Yields
Companion Plants for Better Yields PLANT COMPATIBLE INCOMPATIBLE Angelica Dill Anise Coriander Carrot Black Walnut Tree, Apple Hawthorn Basil, Carrot, Parsley, Asparagus Tomato Azalea Black Walnut Tree Barberry Rye Barley Lettuce Beans, Broccoli, Brussels Sprouts, Cabbage, Basil Cauliflower, Collard, Kale, Rue Marigold, Pepper, Tomato Borage, Broccoli, Cabbage, Carrot, Celery, Chinese Cabbage, Corn, Collard, Cucumber, Eggplant, Irish Potato, Beet, Chive, Garlic, Onion, Beans, Bush Larkspur, Lettuce, Pepper Marigold, Mint, Pea, Radish, Rosemary, Savory, Strawberry, Sunflower, Tansy Basil, Borage, Broccoli, Carrot, Chinese Cabbage, Corn, Collard, Cucumber, Eggplant, Beet, Garlic, Onion, Beans, Pole Lettuce, Marigold, Mint, Kohlrabi Pea, Radish, Rosemary, Savory, Strawberry, Sunflower, Tansy Bush Beans, Cabbage, Beets Delphinium, Onion, Pole Beans Larkspur, Lettuce, Sage PLANT COMPATIBLE INCOMPATIBLE Beans, Squash, Borage Strawberry, Tomato Blackberry Tansy Basil, Beans, Cucumber, Dill, Garlic, Hyssop, Lettuce, Marigold, Mint, Broccoli Nasturtium, Onion, Grapes, Lettuce, Rue Potato, Radish, Rosemary, Sage, Thyme, Tomato Basil, Beans, Dill, Garlic, Hyssop, Lettuce, Mint, Brussels Sprouts Grapes, Rue Onion, Rosemary, Sage, Thyme Basil, Beets, Bush Beans, Chamomile, Celery, Chard, Dill, Garlic, Grapes, Hyssop, Larkspur, Lettuce, Cabbage Grapes, Rue Marigold, Mint, Nasturtium, Onion, Rosemary, Rue, Sage, Southernwood, Spinach, Thyme, Tomato Plant throughout garden Caraway Carrot, Dill to loosen soil Beans, Chive, Delphinium, Pea, Larkspur, Lettuce, -

GREAT-TASTING GINGER ALE with REAL GINGER NOW ONLY 30 CALORIES PER 12Oz
GREAT-TASTING GINGER ALE with REAL GINGER NOW ONLY 30 CALORIES PER 12oz. Real ginger root Dry, spicy taste Naturally sweetened with cane sugar, stevia and monk fruit 30 calories and 6gm sugar per 12oz. No preservatives No caeine Gluten-free Vegan PEACH ORIGINAL DRY RASPBERRY GINGER ALE GINGER ALE GINGER ALE GuS Ginger Ale with Peach is a delightful marriage GuS Original Dry Ginger Ale is crisp and spicy, GuS Ginger Ale with Raspberry is a flavorful blend of real ginger root and refreshing peach. Naturally made from the finest ginger root. Naturally of real ginger root and juicy raspberry. Naturally sweetened and with only 30 calories per 12oz. can, sweetened and with only 30 calories per 12oz. can, sweetened and with only 30 calories per 12oz. can, it’s full of flavor but light on the sugar. it’s full of flavor but light on the sugar. it’s full of flavor but light on the sugar. Nutrition Facts Ingredients: Carbonated water, Nutrition Facts Ingredients: Carbonated water, Nutrition Facts Ingredients: Carbonated water, 1 serving per container 1 serving per container 1 serving per container Serv. Size 12 fl oz (355mL) cane sugar, ginger root extract Serv. Size 12 fl oz (355mL) cane sugar, ginger root extract Serv. Size 12 fl oz (355mL) cane sugar, ginger root extract Amount per serving and other natural flavors, citric Amount per serving and other natural flavors, citric Amount per serving and other natural flavors, citric Calories 30 acid, caramel color, monk fruit Calories 30 acid, caramel color, monk fruit Calories 30 acid, caramel color, monk fruit % Daily Value extract, stevia extract. -

Nutritional and Medicinal Properties of Stevia Rebaudiana
Review Article Curr Res Diabetes Obes J Volume 13 Issue 4 - July 2020 Copyright © All rights are reserved by Fasiha Ahsan DOI: 10.19080/CRDOJ.2020.13.555867 Nutritional and Medicinal Properties of Stevia Rebaudiana Fasiha Ahsan*, Shahid Bashir and Faiz-ul-Hassan Shah University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Submission: June 25, 2020; Published: July 16, 2020 *Corresponding author: Fasiha Ahsan, PhD Scholar, University Institute of Diet and Nutritional Sciences, The University of Lahore, Pakistan Abstract Researches on new molecules with the least toxic effects and better potency is on its way and more attention is being given upon medicinal plants for forcing away the above problems. Medicinal plants have been recognized as potential drug candidates. Stevia, a natural sweetener with medicinal properties and also having nutritional, therapeutic and industrial importance is being used all over the world. Stevia rebaudiana leaves are usually referred to as candy, sweet and honey leaves. Diterpene glycosides are responsible for its high sweetening potential of leaves. The phytochemical properties of bioactive chemicals present in stevia leaves are involves in maintaining the physiological functions of human body. Paper also highlights the importance of nutritional aspects of dried stevia leaves, metabolism of stevia, effects of it consumption on human health and clinical studies related to stevia ingestion. Various medicinal properties of stevia leaves discussed in paper like anti-hyperglycemia, anti-oxidative, hypotensive, nephro-protective, hepato protective, antibacterial and antifungal. Basic purpose of this review to understand the medicinalKeywords: potential Stevia; Diabetes;of stevia and Phytochemicals; its acceptance Medicinal as a significant plant; Steviol;raw material Nutrition; for human Disorders diet. -
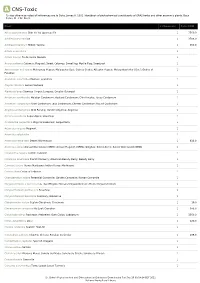
Show Activity
A CNS-Toxic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Achillea moschata Iva 1 3708.0 Achillea millefolium Milfoil; Yarrow 1 550.0 Acinos suaveolens 1 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Calamus; Flagroot; Sweet Calamus; Sweetflag; Myrtle Flag; Sweetroot 1 Aframomum melegueta Melegueta Pepper; Malagueta (Sp.); Guinea Grains; Alligator Pepper; Malagettapfeffer (Ger.); Grains-of- 1 Paradise Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 Amomum xanthioides Malabar Cardamom; Bastard Cardamom; Chin Kousha; Tavoy Cardamom 1 Amomum compactum Siam Cardamom; Java Cardamom; Chester Cardamom; Round Cardamom 1 Angelica archangelica Wild Parsnip; Garden Angelica; Angelica 1 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Virginia Snakeroot; Serpentaria 1 Artemisia vulgaris Mugwort 1 Artemisia salsoloides 1 Artemisia herba-alba Desert Wormwood 1 638.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Qinghao; Sweet Annie; Sweet Wormwood (GRIN) 1 Calamintha nepeta Turkish Calamint 1 Callicarpa americana French Mulberry; American Beauty Berry; Beauty Berry 1 Cannabis sativa Hemp; Marijuana; Indian Hemp; Marihuana 1 Cedrus libani Cedar of Lebanon 1 Chamaemelum nobile Perennial Camomile; Garden Camomile; Roman Camomile