Aspects of Antipredation in Panulirus Argus and Panulirus Guttatus: Behavior, Morphology, and Ontogeny Peter Edward Bouwma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Classification of Living and Fossil Genera of Decapod Crustaceans
RAFFLES BULLETIN OF ZOOLOGY 2009 Supplement No. 21: 1–109 Date of Publication: 15 Sep.2009 © National University of Singapore A CLASSIFICATION OF LIVING AND FOSSIL GENERA OF DECAPOD CRUSTACEANS Sammy De Grave1, N. Dean Pentcheff 2, Shane T. Ahyong3, Tin-Yam Chan4, Keith A. Crandall5, Peter C. Dworschak6, Darryl L. Felder7, Rodney M. Feldmann8, Charles H. J. M. Fransen9, Laura Y. D. Goulding1, Rafael Lemaitre10, Martyn E. Y. Low11, Joel W. Martin2, Peter K. L. Ng11, Carrie E. Schweitzer12, S. H. Tan11, Dale Tshudy13, Regina Wetzer2 1Oxford University Museum of Natural History, Parks Road, Oxford, OX1 3PW, United Kingdom [email protected] [email protected] 2Natural History Museum of Los Angeles County, 900 Exposition Blvd., Los Angeles, CA 90007 United States of America [email protected] [email protected] [email protected] 3Marine Biodiversity and Biosecurity, NIWA, Private Bag 14901, Kilbirnie Wellington, New Zealand [email protected] 4Institute of Marine Biology, National Taiwan Ocean University, Keelung 20224, Taiwan, Republic of China [email protected] 5Department of Biology and Monte L. Bean Life Science Museum, Brigham Young University, Provo, UT 84602 United States of America [email protected] 6Dritte Zoologische Abteilung, Naturhistorisches Museum, Wien, Austria [email protected] 7Department of Biology, University of Louisiana, Lafayette, LA 70504 United States of America [email protected] 8Department of Geology, Kent State University, Kent, OH 44242 United States of America [email protected] 9Nationaal Natuurhistorisch Museum, P. O. Box 9517, 2300 RA Leiden, The Netherlands [email protected] 10Invertebrate Zoology, Smithsonian Institution, National Museum of Natural History, 10th and Constitution Avenue, Washington, DC 20560 United States of America [email protected] 11Department of Biological Sciences, National University of Singapore, Science Drive 4, Singapore 117543 [email protected] [email protected] [email protected] 12Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Mississippi-Alabama Sea Grant Consortium 2018-21 Strategic Plan Table of Contents Strategic Planning Process
Mississippi-Alabama Sea Grant Consortium 2018-21 Strategic Plan Table of Contents Strategic Planning Process .............................................................................................................. 1 External and Internal Input ......................................................................................................... 1 Vision ............................................................................................................................................... 3 Mission ............................................................................................................................................ 3 Core Values ..................................................................................................................................... 3 Organizational Excellence ............................................................................................................... 3 Partnerships .................................................................................................................................... 4 Shared Positions While Leveraging Partnerships ........................................................................ 4 Gulf Sea Grant Programs ............................................................................................................. 4 State and Local Agencies ............................................................................................................. 5 Federal Agencies ........................................................................................................................ -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -

Factors Affecting Growth of the Spiny Lobsters Panulirus Gracilis and Panulirus Inflatus (Decapoda: Palinuridae) in Guerrero, México
Rev. Biol. Trop. 51(1): 165-174, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Factors affecting growth of the spiny lobsters Panulirus gracilis and Panulirus inflatus (Decapoda: Palinuridae) in Guerrero, México Patricia Briones-Fourzán and Enrique Lozano-Álvarez Universidad Nacional Autónoma de México, Instituto de Ciencias del Mar y Limnología, Unidad Académica Puerto Morelos. P. O. Box 1152, Cancún, Q. R. 77500 México. Fax: +52 (998) 871-0138; [email protected] Received 00-XX-2002. Corrected 00-XX-2002. Accepted 00-XX-2002. Abstract: The effects of sex, injuries, season and site on the growth of the spiny lobsters Panulirus gracilis, and P. inflatus, were studied through mark-recapture techniques in two sites with different ecological characteristics on the coast of Guerrero, México. Panulirus gracilis occurred in both sites, whereas P. inflatus occurred only in one site. All recaptured individuals were adults. Both species had similar intermolt periods, but P. gracilis had significantly higher growth rates (mm carapace length week-1) than P. inflatus as a result of a larger molt incre- ment. Growth rates of males were higher than those of females in both species owing to larger molt increments and shorter intermolt periods in males. Injuries had no effect on growth rates in either species. Individuals of P. gracilis grew faster in site 1 than in site 2. Therefore, the effect of season on growth of P. gracilis was analyzed separately in each site. In site 2, growth rates of P. gracilis were similar in summer and in winter, whereas in site 1 both species had higher growth rates in winter than in summer. -
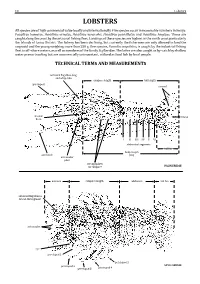
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

The Twenty-One Member Schools of the Dauphin Island Sea Lab/ Marine Environmental Sciences Consortium
Dauphin Island Sea Lab Alabamaʼs Marine Science Education and Research Institution 1975 1975 2006 2006 Annual Report The Twenty-one Member Schools of the Dauphin Island Sea Lab/ Marine Environmental Sciences Consortium • Alabama State University, Montgomery, AL* • Athens State University, Athens, AL • Auburn University, Auburn, AL* • Auburn University at Montgomery, Montgomery, AL • Birmingham Southern College, Birmingham, AL • Huntingdon College, Montgomery, AL • Jacksonville State University, Jacksonville, AL* • Judson College, Marion, AL • Samford University, Birmingham, AL* • Spring Hill College, Mobile, AL • Talladega College, Talladega, AL • Troy University, Troy, AL • Tuskegee University, Tuskegee, AL* • University of Alabama, Tuscaloosa, AL* • University of Alabama at Birmingham, Birmingham, AL* • University of Alabama in Huntsville, Huntsville, AL* • University of Mobile, Mobile, AL • University of Montevallo, Montevallo, AL • University of North Alabama, Florence, AL • University of South Alabama, Mobile, AL* • University of West Alabama, Livingston, AL * Schools with Graduate Degree Programs Annual Report 2006 - page 2 Statement of Purpose The Dauphin Island Sea Lab (DISL) is Alabama’s marine research and educational institution. Founded in 1971 by the Alabama legislature to maximize the marine sciences capabilities of several Alabama institutions and minimize duplication, DISL serves twenty-one Alabama colleges and universities, both public and private. DISL and its faculty work toward the combined purposes of conducting pure -

Drivers of Octopus Abundance in an Anchialine Lake
1 1 Drivers of octopus abundance and density in an anchialine lake: 2 a 30 year comparison 3 4 Duncan A. O’Brien a,d* - [email protected] 5 Michelle L. Taylor a - [email protected] 6 Heather D. Masonjones b - [email protected] 7 Philipp H. Boersch-Supan c - [email protected] 8 Owen R. O’Shea d - [email protected] 9 10 a School of Life Sciences, University of Essex, Wivenhoe Park, Colchester, CO4 3SQ, UK 11 b Biology Department, University of Tampa, Tampa, Florida 33596, USA 12 c British Trust for Ornithology, The Nunnery, Thetford, IP24 2PU, UK and Department of 13 Geography, University of Florida, Gainesville, Florida 32611, USA 14 d The Centre for Ocean Research and Education (CORE), PO Box 255-16, Gregory Town, 15 Eleuthera, The Bahamas 16 17 *Corresponding author 18 19 20 21 22 23 24 25 26 2 27 Abstract 28 Anchialine systems are isolated from the sea and often support species’ populations distinct from 29 their marine counterparts. Sweetings Pond, an anchialine lake on the island of Eleuthera in The 30 Bahamas was identified as a site of high Caribbean reef octopus, Octopus briareus (Robson, 31 1929) density, relative to coastal populations. However, observed deterioration in local benthic 32 habitat and increased anthropogenic influence over the last 30 years imply that this octopus 33 population may have undergone density and distribution shifts in response to these changing 34 conditions. Here, we assess the system wide octopus density to provide an updated estimate. We 35 hypothesize that despite depressed habitat availability in the 1980s, it will now support octopus 36 densities less than historical estimates because of increasing human impact on the system. -

Mimicry and Defense
3/24/2015 Professor Donald McFarlane Mimicry and Defense Protective Strategies Camouflage (“Cryptic coloration”) Diverse Coloration Diversion Structures Startle Structures 2 1 3/24/2015 Camouflage (“Cryptic coloration”) Minimize 3d shape, e.g. flatfish Halibut (Hippoglossus hippoglossus) 3 4 2 3/24/2015 Counter‐Shading 5 Disruptive Coloration 6 3 3/24/2015 Polymorphism – Cepeae snails 7 Polymorphism – Oophaga granuliferus 8 4 3/24/2015 Polymorphism – 9 Polymorphism – Oophaga Geographic locations of study populations and their color patterns. (A) Map of the pacific coast of Colombia showing the three study localities: in blue Oophaga histrionica, in orange O. lehmanni, and in green the pHYB population. (B) Examples of color patterns of individuals from the pHYB population (1–4) and the pattern from a hybrid between Oophaga histrionica and O. lehmanni bred in the laboratory (H) 10 5 3/24/2015 Diversion Structures 11 Startle Structures 12 6 3/24/2015 Warning Coloration (Aposematic coloration) Advertise organism as distasteful, toxic or venomous Problem: Predators must learn by attacking prey; predator learning is costly to prey. Therefore strong selective pressure to STANDARDIZE on a few colors/patterns. This is MULLERIAN MIMICRY. Most common is yellow/black, or red/yellow/black 13 Warning Coloration (Aposematic coloration) Bumblebee (Bombus Black and yellow mangrove snake (Boiga sp.) Sand Wasp (bembix oculata) dendrophila) Yellow‐banded poison dart frog (Dendrobates leucomelas Fire salamander ( Salamandra salamandra) 14 7 3/24/2015 Warning Coloration (Aposematic coloration) coral snakes (Micrurus sp.) ~ 50 species in two families, all venomous 15 Batesian Mimicry 1862 –Henry Walter Bates; “A Naturalist on the River Amazons” 16 8 3/24/2015 Batesian Mimicry Batesian mimics “cheat” –they lack toxins, venom, etc. -

Environmental Monitors on Lobster Traps Phase VII: Validating Ocean Models
Environmental Monitors on Lobster Traps Phase VII: Validating Ocean Models Progress Report February 2011 Award number: 07-051 Period of performance: 06/30/08-1/15/10 Date of progress report submission: February 2011 Final report due: June 2011 Contact information of the principal investigator: Jim Manning NOAA/NEFSC 166 Water St Woods Hole, MA 02543 508-495-2211 [email protected] Other key participants: Erin Pelletier, 207-985-8088, [email protected] Gulf of Maine Lobster Foundation Vitalii Sheremet, [email protected] University of Rhode Island Dave Casoni, 508-224-3038, [email protected] Massachusetts Lobstermen Major accomplishments and milestones: Considerable advances have been made towards the validation of local ocean models in the last few years. Given new utilities that allow investigators to remotely access a variety of web-served model output, it is now possible to examine these models without needing to bother the modeling teams that generate the output. These are powerful new tools that can be leveraged. Because of the activity associated with this NEC-funded grant, I was invited to sit on a advisory panel that evaluates UMASSS Dartmouth©s FVCOM model operations: the Northeast Coastal Ocean Forecast System. Much of the work that has been done and the tools that have been develop in this grant therefore have addressed the FVCOM model in particular. However, there are multiple models that simulate our coastal waters and these tools have been applied to these other models as well. The validation of models has progressed along a few fronts associated with different data products. -

Feeding Habits of the Prawns Processa Edulzs and Palaemon Adspersus (Crustacea, Decapoda, Caridea) in the Alfacs Bay, Ebro Delta (Nw Mediterranean)
FEEDING HABITS OF THE PRAWNS PROCESSA EDULZS AND PALAEMON ADSPERSUS (CRUSTACEA, DECAPODA, CARIDEA) IN THE ALFACS BAY, EBRO DELTA (NW MEDITERRANEAN) Guerao, G., 1993-1994. Feeding habits of the prawns Processa edulis and Palaemon adspersus (Crustacea, Decapoda, Caridea) in the Alfacs Bay, Ebro Delta (NW Mediterranean). Misc. Zool., 17: 115-122. Feeding habits of the prawns Processa edulis and Palaemon adspersus (Crustacea, Decapoda, Caridea) in the Alfacs Bay, Ebro Delta (NW Mediterranean).- The stomach contents of 147 Palaemon adspersus Rathke, and 102 Processa edulis (Risso) were analyzed. The frequency of occurrence method and the points method were used. The role of these species in the food web of Cymodocea nodosa meadows is defined. Results indicate that both species are predators of benthic invertebrates rather than scavengers or detritus feeders. The main food items varied according to species. The diet of Palaemon adspersus consisted almost entirely of crustaceans, molluscs, and plant material, with amphipods playing a major role. Processa edulis ate an almost equal amount of crustaceans and polychaetes. In P. adspersus, most dietary items differed according to size classes of prawn. Key words: Feeding, Prawns, Palaemon, Processa, Ebro Delta. (Rebut: 18 V 94; Acceptació condicional: 13 IX 94; Acc. definitiva: 18 X 94) G. Guerao, Dept. de Biologia Animal (Artrdpodes), Fac. de Biologia, Univ. de Barcelona, Avgda. Diagonal 645, 08028 Barcelona, Espanya (Spain). INTRODUCTION and has been recorded from as far north as the Norwegian Sea to the Marocco coast Processidae prawns are abundant in coastal (LAGARDERE,1971) and the Mediterranean waters of temperate and tropical areas. (ZARIQUIEYÁLVAREZ, 1968). This species is Processa edulis (Risso, 1816) is a comrnon the subject of commercial fisheries in many littoral mediterranean prawn (ZARIQUIEY areas (JENSEN,1958; HOLTHUIS,1980; ÁLVAREZ,1968). -

Müllerian and Batesian Mimicry Rings of White- Variegated Aposematic Spiny and Thorny Plants: a Hypothesis
Israel Journal of Plant Sciences ISSN: 0792-9978 (Print) 2223-8980 (Online) Journal homepage: http://www.tandfonline.com/loi/tips20 Müllerian and Batesian mimicry rings of white- variegated aposematic spiny and thorny plants: A hypothesis Simcha Lev-Yadun To cite this article: Simcha Lev-Yadun (2009) Müllerian and Batesian mimicry rings of white- variegated aposematic spiny and thorny plants: A hypothesis, Israel Journal of Plant Sciences, 57:1-2, 107-116 To link to this article: http://dx.doi.org/10.1560/IJPS.57.1-2.107 Published online: 14 Mar 2013. Submit your article to this journal Article views: 41 View related articles Citing articles: 1 View citing articles Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tips20 Download by: [Universitaire De Lausanne] Date: 03 May 2016, At: 02:12 Israel Journal of Plant Sciences Vol. 57 2009 pp. 107–116 DOI: 10.1560/IJPS.57.1–2.107 This paper has been contributed in honor of Azaria Alon on the occasion of his 90th birthday. Müllerian and Batesian mimicry rings of white-variegated aposematic spiny and thorny plants: A hypothesis SIMCHA LEV-YADUN Department of Science Education–Biology, Faculty of Science and Science Education, University of Haifa—Oranim, Tivon 36006, Israel (Received 4 August 2008; accepted in revised form 9 March 2009) ABSTRACT Twenty-one wild spiny or thorny plant species growing in Israel have been found so far that are conspicuous because of white stripes and spots found on their leaves. Twenty of these species occupy open habitats, and only one is a climber (Smilax aspera) that is found in both shady and open habitats.