Understanding Tracking Detectors That Rely on Ionisation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Great Physicists
Great Physicists Great Physicists The Life and Times of Leading Physicists from Galileo to Hawking William H. Cropper 1 2001 1 Oxford New York Athens Auckland Bangkok Bogota´ Buenos Aires Cape Town Chennai Dar es Salaam Delhi Florence HongKong Istanbul Karachi Kolkata Kuala Lumpur Madrid Melbourne Mexico City Mumbai Nairobi Paris Sao Paulo Shanghai Singapore Taipei Tokyo Toronto Warsaw and associated companies in Berlin Ibadan Copyright ᭧ 2001 by Oxford University Press, Inc. Published by Oxford University Press, Inc. 198 Madison Avenue, New York, New York 10016 Oxford is a registered trademark of Oxford University Press All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of Oxford University Press. Library of Congress Cataloging-in-Publication Data Cropper, William H. Great Physicists: the life and times of leadingphysicists from Galileo to Hawking/ William H. Cropper. p. cm Includes bibliographical references and index. ISBN 0–19–513748–5 1. Physicists—Biography. I. Title. QC15 .C76 2001 530'.092'2—dc21 [B] 2001021611 987654321 Printed in the United States of America on acid-free paper Contents Preface ix Acknowledgments xi I. Mechanics Historical Synopsis 3 1. How the Heavens Go 5 Galileo Galilei 2. A Man Obsessed 18 Isaac Newton II. Thermodynamics Historical Synopsis 41 3. A Tale of Two Revolutions 43 Sadi Carnot 4. On the Dark Side 51 Robert Mayer 5. A Holy Undertaking59 James Joule 6. Unities and a Unifier 71 Hermann Helmholtz 7. The Scientist as Virtuoso 78 William Thomson 8. -

Curren T Anthropology
Forthcoming Current Anthropology Wenner-Gren Symposium Curren Supplementary Issues (in order of appearance) t Humanness and Potentiality: Revisiting the Anthropological Object in the Anthropolog Current Context of New Medical Technologies. Klaus Hoeyer and Karen-Sue Taussig, eds. Alternative Pathways to Complexity: Evolutionary Trajectories in the Anthropology Middle Paleolithic and Middle Stone Age. Steven L. Kuhn and Erella Hovers, eds. y THE WENNER-GREN SYMPOSIUM SERIES Previously Published Supplementary Issues December 2012 HUMAN BIOLOGY AND THE ORIGINS OF HOMO Working Memory: Beyond Language and Symbolism. omas Wynn and Frederick L. Coolidge, eds. GUEST EDITORS: SUSAN ANTÓN AND LESLIE C. AIELLO Engaged Anthropology: Diversity and Dilemmas. Setha M. Low and Sally Early Homo: Who, When, and Where Engle Merry, eds. Environmental and Behavioral Evidence V Dental Evidence for the Reconstruction of Diet in African Early Homo olum Corporate Lives: New Perspectives on the Social Life of the Corporate Form. Body Size, Body Shape, and the Circumscription of the Genus Homo Damani Partridge, Marina Welker, and Rebecca Hardin, eds. Ecological Energetics in Early Homo e 5 Effects of Mortality, Subsistence, and Ecology on Human Adult Height 3 e Origins of Agriculture: New Data, New Ideas. T. Douglas Price and Plasticity in Human Life History Strategy Ofer Bar-Yosef, eds. Conditions for Evolution of Small Adult Body Size in Southern Africa Supplement Growth, Development, and Life History throughout the Evolution of Homo e Biological Anthropology of Living Human Populations: World Body Size, Size Variation, and Sexual Size Dimorphism in Early Homo Histories, National Styles, and International Networks. Susan Lindee and Ricardo Ventura Santos, eds. -

A Brief History of Microwave Engineering
A BRIEF HISTORY OF MICROWAVE ENGINEERING S.N. SINHA PROFESSOR DEPT. OF ELECTRONICS & COMPUTER ENGINEERING IIT ROORKEE Multiple Name Symbol Multiple Name Symbol 100 hertz Hz 101 decahertz daHz 10–1 decihertz dHz 102 hectohertz hHz 10–2 centihertz cHz 103 kilohertz kHz 10–3 millihertz mHz 106 megahertz MHz 10–6 microhertz µHz 109 gigahertz GHz 10–9 nanohertz nHz 1012 terahertz THz 10–12 picohertz pHz 1015 petahertz PHz 10–15 femtohertz fHz 1018 exahertz EHz 10–18 attohertz aHz 1021 zettahertz ZHz 10–21 zeptohertz zHz 1024 yottahertz YHz 10–24 yoctohertz yHz • John Napier, born in 1550 • Developed the theory of John Napier logarithms, in order to eliminate the frustration of hand calculations of division, multiplication, squares, etc. • We use logarithms every day in microwaves when we refer to the decibel • The Neper, a unitless quantity for dealing with ratios, is named after John Napier Laurent Cassegrain • Not much is known about Laurent Cassegrain, a Catholic Priest in Chartre, France, who in 1672 reportedly submitted a manuscript on a new type of reflecting telescope that bears his name. • The Cassegrain antenna is an an adaptation of the telescope • Hans Christian Oersted, one of the leading scientists of the Hans Christian Oersted nineteenth century, played a crucial role in understanding electromagnetism • He showed that electricity and magnetism were related phenomena, a finding that laid the foundation for the theory of electromagnetism and for the research that later created such technologies as radio, television and fiber optics • The unit of magnetic field strength was named the Oersted in his honor. -

John Napier's Life Napier's Logarithms KYLIE BRYANT & PAUL SCOTT
KYLIE BRYANT & PAUL SCOTT John Napier’s life Napier’s logarithms John Napier was born in 1550 in the Tower of Napier’s idea in inventing the logarithm was to Merchiston, near Edinburgh, Scotland. His save mathematicians from having to do large father, Archibald Napier, was knighted in 1565 calculations. These days, calculations and was appointed Master of the Mint in 1582. involving large numbers are done using calcu- His mother, Janet Bothwell, was the sister of lators or computers; however before these were the Bishop of Orkney. Napier’s was an impor- invented, large calculations meant that much tant family in Scotland, and had owned the time was taken and mistakes were made. Merchiston estate since 1430. Napier’s logarithm was not defined in terms Napier was educated at St Salvator’s of exponents as our logarithm is today. College, graduating in 1563. He was just 13 Instead Napier thought of his logarithm in years old when his mother died and he was terms of moving particles, distances and veloc- sent to St Andrew’s University where he ities. He considered two lines, AZ of fixed studied for two years. This is where he gained length and A'Z' of infinite length and points X his interest in theology. He did not graduate, and X' starting at A and A' moving to the right however, instead leaving to travel around with the same initial velocity. X' has constant Europe for the next five years. During this velocity and X has a velocity proportional to time he gained knowledge of mathematics and the distance from X to Z. -

The Logarithmic Tables of Edward Sang and His Daughters
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Historia Mathematica 30 (2003) 47–84 www.elsevier.com/locate/hm The logarithmic tables of Edward Sang and his daughters Alex D.D. Craik School of Mathematics & Statistics, University of St Andrews, St Andrews, Fife KY16 9SS, Scotland, United Kingdom Abstract Edward Sang (1805–1890), aided only by his daughters Flora and Jane, compiled vast logarithmic and other mathematical tables. These exceed in accuracy and extent the tables of the French Bureau du Cadastre, produced by Gaspard de Prony and a multitude of assistants during 1794–1801. Like Prony’s, only a small part of Sang’s tables was published: his 7-place logarithmic tables of 1871. The contents and fate of Sang’s manuscript volumes, the abortive attempts to publish them, and some of Sang’s methods are described. A brief biography of Sang outlines his many other contributions to science and technology in both Scotland and Turkey. Remarkably, the tables were mostly compiled in his spare time. 2003 Elsevier Science (USA). All rights reserved. Résumé Edward Sang (1805–1890), aidé seulement par sa famille, c’est à dire ses filles Flora et Jane, compila des tables vastes des logarithmes et des autres fonctions mathématiques. Ces tables sont plus accurates, et plus extensives que celles du Bureau du Cadastre, compileés les années 1794–1801 par Gaspard de Prony et une foule de ses aides. On ne publia qu’une petite partie des tables de Sang (comme celles de Prony) : ses tables du 1871 des logarithmes à 7-places décimales. -

November 2019
A selection of some recent arrivals November 2019 Rare and important books & manuscripts in science and medicine, by Christian Westergaard. Flæsketorvet 68 – 1711 København V – Denmark Cell: (+45)27628014 www.sophiararebooks.com AMPÈRE, André-Marie. THE FOUNDATION OF ELECTRO- DYNAMICS, INSCRIBED BY AMPÈRE AMPÈRE, Andre-Marie. Mémoires sur l’action mutuelle de deux courans électri- ques, sur celle qui existe entre un courant électrique et un aimant ou le globe terres- tre, et celle de deux aimans l’un sur l’autre. [Paris: Feugeray, 1821]. $22,500 8vo (219 x 133mm), pp. [3], 4-112 with five folding engraved plates (a few faint scattered spots). Original pink wrappers, uncut (lacking backstrip, one cord partly broken with a few leaves just holding, slightly darkened, chip to corner of upper cov- er); modern cloth box. An untouched copy in its original state. First edition, probable first issue, extremely rare and inscribed by Ampère, of this continually evolving collection of important memoirs on electrodynamics by Ampère and others. “Ampère had originally intended the collection to contain all the articles published on his theory of electrodynamics since 1820, but as he pre- pared copy new articles on the subject continued to appear, so that the fascicles, which apparently began publication in 1821, were in a constant state of revision, with at least five versions of the collection appearing between 1821 and 1823 un- der different titles” (Norman). The collection begins with ‘Mémoires sur l’action mutuelle de deux courans électriques’, Ampère’s “first great memoir on electrody- namics” (DSB), representing his first response to the demonstration on 21 April 1820 by the Danish physicist Hans Christian Oersted (1777-1851) that electric currents create magnetic fields; this had been reported by François Arago (1786- 1853) to an astonished Académie des Sciences on 4 September. -

The Mathematical Work of John Napier (1550-1617)
BULL. AUSTRAL. MATH. SOC. 0IA40, 0 I A45 VOL. 26 (1982), 455-468. THE MATHEMATICAL WORK OF JOHN NAPIER (1550-1617) WILLIAM F. HAWKINS John Napier, Baron of Merchiston near Edinburgh, lived during one of the most troubled periods in the history of Scotland. He attended St Andrews University for a short time and matriculated at the age of 13, leaving no subsequent record. But a letter to his father, written by his uncle Adam Bothwell, reformed Bishop of Orkney, in December 1560, reports as follows: "I pray you Sir, to send your son John to the Schools either to France or Flanders; for he can learn no good at home, nor gain any profit in this most perilous world." He took an active part in the Reform Movement and in 1593 he produced a bitter polemic against the Papacy and Rome which was called The Whole Revelation of St John. This was an instant success and was translated into German, French and Dutch by continental reformers. Napier's reputation as a theologian was considerable throughout reformed Europe, and he would have regarded this as his chief claim to scholarship. Throughout the middle ages Latin was the medium of communication amongst scholars, and translations into vernaculars were the exception until the 17th and l8th centuries. Napier has suffered badly through this change, for up till 1889 only one of his four works had been translated from Latin into English. Received 16 August 1982. Thesis submitted to University of Auckland, March 1981. Degree approved April 1982. Supervisors: Mr Garry J. Tee Professor H.A. -

SIS Bulletin Issue 56
Scientific Instrument Society Bulletin March No. 56 1998 Bulletin of the Scientific Instrument Society tSSN09S6-s271 For Table of Contents, see back cover President Gerard Turner Vice.President Howard Dawes Honorary Committee Stuart Talbot, Chairman Gloria Clifton,Secretary John Didcock, Treasurer Willern Hackrnann, Editor Jane Insley,Adzwtzsmg Manager James Stratton,Meetings Secreta~. Ron Bnstow Alexander Crum-Ewing Colin Gross Arthur Middleton Liba Taub Trevor Waterman Membership and Administrative Matters The Executive Officer (Wg Cdr Geofl~,V Bennett) 31 High Street Stanford in the Vale Faringdon Tel: 01367 710223 OxOn SN7 8LH Fax: 01367 718963 e-mail: [email protected] See outside back cover for infvrmatam on membership Editorial Matters Dr. Willem D. Hackmann Museum of the History of Science Old Ashmolean Building Tel: 01865 277282 (office) Broad Street Fax: 01865 277288 Oxford OXl 3AZ Tel: 016~ 811110 (home) e-mail: willem.hac~.ox.ac.uk Society's Website http://www.sis.org.uk Advertising Jane lnsley Science Museum Tel: 0171-938 8110 South Kensington Fax: 0171-938 8118 London SW7 2DD e-mail: j.ins~i.ac.uk Organization of Meetings Mr James Stratton 101 New Bond Street Tel: 0171-629 2344 l.xmdon WIY 0AS Fax: 0171-629 8876 Typesetting and Printing Lahoflow Ltd 26-~ Wharfdale Road Tel: 0171-833 2344 King's Cross Fax: 0171-833 8150 L~mdon N! 9RY e-mail: lithoflow.co.uk Price: ~ per issue, uncluding back numbers where available. (Enquiries to the Executive Off-a:er) The Scientific Instrument Society is Registered Charity No. 326733 © The ~:~t~ L~n~.nt Society l~ Editorial l'idlil~iil,lo ~If. -

History in the Computing Curriculum 6000 BC to 1899 AD
History in the Computing Curriculum Appendix A1 6000 BC to 1899 AD 6000 B.C. [ca]: Ishango bone type of tally stick in use. (w) 4000-1200 B.C.: Inhabitants of the first known civilization in Sumer keep records of commercial transactions on clay tablets. (e) 3000 B.C.: The abacus is invented in Babylonia. (e) 1800 B.C.: Well-developed additive number system in use in Egypt. (w) 1300 B.C.: Direct evidence exists as to the Chinese using a positional number system. (w) 600 B.C. [ca.]: Major developments start to take place in Chinese arithmetic. (w) 250-230 B.C.: The Sieve of Eratosthenes is used to determine prime numbers. (e) 213 B.C.: Chi-Hwang-ti orders all books in China to be burned and scholars to be put to death. (w) 79 A.D. [ca.]: "Antikythera Device," when set correctly according to latitude and day of the week, gives alternating 29- and 30-day lunar months. (e) 800 [ca.]: Chinese start to use a zero, probably introduced from India. (w) 850 [ca.]: Al-Khowarizmi publishes his "Arithmetic." (w) 1000 [ca.]: Gerbert describes an abacus using apices. (w) 1120: Adelard of Bath publishes "Dixit Algorismi," his translation of Al-Khowarizmi's "Arithmetic." (w) 1200: First minted jetons appear in Italy. (w) 1202: Fibonacci publishes his "Liber Abaci." (w) 1220: Alexander De Villa Dei publishes "Carmen de Algorismo." (w) 1250: Sacrobosco publishes his "Algorismus Vulgaris." (w) 1300 [ca.]: Modern wire-and-bead abacus replaces the older Chinese calculating rods. (e,w) 1392: Geoffrey Chaucer publishes the first English-language description on the uses of an astrolabe. -
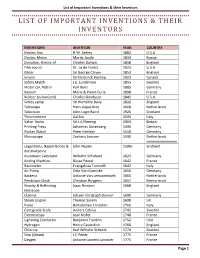
List of Important Inventions & Their Inventors
List of Important Inventions & their Inventors LIST OF IMPORTANT INVENTIONS & THEIR INVENTORS INVENTIONS INVENTOR YEAR COUNTRY Electric Iron H.W. Seeley 1882 U.S.A Electric Motor Moritz Jacobi 1834 Russia Evolution, theory of Charles Darwin 1858 England Film sound Dr. Le de Forest 1923 U.S.A Glider Sir George Calyey 1853 England Insulin Sir Frederick Banting 1923 Canada Safety Match J.E. Lundstrom 1855 Sweden Motor car, Petrol Karl Benz 1885 Germany Radium Marie & Pierre Curie 1898 France Rubber (vulcanized) Charles Goodyear 1841 U.S.A Safety Lamp Sir Humphry Davy 1816 England Telescope Hans Lippershey 1608 Netherlands Television John Logic Baird 1926 Scotland Thermometer Galileo 1593 Italy Valve. Radio Sir J.A Fleming 1904 Britain Printing Press Johannes Gutenberg 1440 Germany Pocket Watch Peter Henlein 1510 Germany Microscope Zacharis Janssen 1590 Netherlands Logarithms, Napier Bones & John Napier 1590s Scotland decimal point Automatic Calculator Wilhelm Schickard 1623 Germany Adding Machine Blaise Pascal 1642 France Barometer Evangelista Torricelli 1643 Italy Air Pump Otto Von Guericke 1650 Germany Bacteria Antonie Van Leeuwenhoek 1665 Netherlands Pendulum Clock Christian Huygens 1657 Netherlands Gravity & Reflecting Isaac Newton 1668 England telescope Clarinet Johann Christoph Denner 1690 Germany Steam Engine Thomas Savery 1698 UK Piano Bartolomeo Cristofori 1700 Italy Centigrade Scale Anders Celsius 1742 Sweden Electroscope Jean Nollet 1748 France Lightning Conductor Benjamin Franklin 1752 USA Hydrogen Henry Cavendish 1766 England Chlorine Karl Wilhelm Scheele 1774 Sweden Ship (Steam) J.C Perier 1775 France Oxygen Antoine Laurent Lavoisier 1775 France Page 1 List of Important Inventions & their Inventors Submarine David Bushnell 1776 USA Hot Air Balloon Josef & Etienne Montgolfier 1783 France Tungsten Juan José Elhuyar Lubize & 1783 Spain Fausto de Elhuyar Bifocal Lens Benjamin Franklin 1784 USA Parachute Jean Pierre Blanchard 1785 France Steam Boat John Fitch 1786 USA Guillotin Dr. -
Timothy Foster, Bryan Johnson, Travis Eckstrom, Andrew Sutter • Indicated by the Function 푦 = 푓−1 푥 , Inversely Related to 푦 = 푓(푥)
Timothy Foster, Bryan Johnson, Travis Eckstrom, Andrew Sutter • Indicated by the function 푦 = 푓−1 푥 , inversely related to 푦 = 푓(푥). • Domain of the inverse corresponds to the Range of the original function, and vice versa. • Can be shown as a reflection across 푦 = 푥 • Indicated by the Function 푓 푥 = 푎푥 such that 푎 is a positive number. • Used for modeling Exponential Growth/Decay, or Continuous Compound Interest • Inverse of exponential function; 푥 if 푓 푥 = 푎 , 푥 = 푙표푔푎푓(푥) • Logarithm with base 푒 known as “natural logarithm”, represented by 푙푛 The idea that a mathematical inverse to exponential growth and decay existed had been understood even by the earliest of mathematicians. However, it was not until the early 1600s that the principles of logarithms were published by John Napier of Scotland. The availability of Logarithms opened up new frontiers in mathematics, while simplifying old formulas. This advancement took place due to the fact that previously difficult multiplications and divisions could be represented by the addition or subtraction of logarithms due to the law of exponents. • Key term: One-to-one Function- A function in which there are no repeated x values for any given y value. • Given a graph, the best way to determine whether or not a function is one-to-one is to perform the “horizontal line test,” which is similar to the “vertical line test” for determining if a graph represents a function. An example of the horizontal line test is shown on the next slide: 2 y=(x-2) y=[(x-3)3+8]/4 4 4 3 3 2 2 1 1 0 Y 0 0 1 2 3 4 Y 0 1 2 3 4 X X Since the graph intercepts the For each y value, there is only horizontal line at multiple one corresponding x value; the points, the function is not function graphed is one-to- considered one-to-one. -

University-Industry Partnership in the Vanguard of Knowledge-Driven Economy Others As Dialectic/Logic, Grammar, Rhetoric Belonged to Trivium (Three Programs)
Journal of Modern Education Review, ISSN 2155-7993, USA July 2014, Volume 4, No. 7, pp. 480–509 Doi: 10.15341/jmer(2155-7993)/07.04.2014/002 Academic Star Publishing Company, 2014 http://www.academicstar.us University-industry Partnership in the Vanguard of Knowledge-Driven Economy László Szentirmai, László Radács (Department of Electrical and Electronic Engineering, University of Miskolc, Hungary) Abstract: The words volt, ampere and watt are named after emblematic figures of university and industry and are in common parlance among people. And what is more, the current International System of Units gave many great names to derived measuring units. The 5–10 year-long strategic partnership is top priority for elite universities. This most creative and promising collaboration ensures big and common strategic goals, and shared research vision. New knowledge generated by alliance modernizes university role and industry long-term strategy. Diverse engineering culture serves as brake but could be overcome by both parties when criterion is excellence. Trend is twofold: either richer benefits are given to fewer universities and/or universities in emerging economies are also to flourish. Operational partnerships — the second type — are based on joint research projects utilizing both the core academic strengths of universities and the core research competence of industry. Research projects on professors’ own initiatives and students’ individual research projects all pave the way for achieving strategic partnership. Transactional partnership — the third type — puts significant impact on teaching and learning. Key criteria include multidisciplinary institute on campus with industry contribution, even culture and curriculum and multidisciplinary approach to research. These criteria together with industrial practice for academics and students lead also this link to strategic collaboration.