Curren T Anthropology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Evolution of Infanticide by Females in Mammals Dieter Lukas, Elise Huchard
The evolution of infanticide by females in mammals Dieter Lukas, Elise Huchard To cite this version: Dieter Lukas, Elise Huchard. The evolution of infanticide by females in mammals. Philosophical Transactions of the Royal Society B: Biological Sciences, Royal Society, The, 2019, 10.1101/405688. hal-02114584 HAL Id: hal-02114584 https://hal.archives-ouvertes.fr/hal-02114584 Submitted on 29 Apr 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. bioRxiv preprint first posted online Aug. 31, 2018; doi: http://dx.doi.org/10.1101/405688. The copyright holder for this preprint (which was not peer-reviewed) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license. The evolution of infanticide by females in mammals Dieter Lukas1,2* & Elise Huchard1,3 1) Department of Zoology, University of Cambridge, Downing Street, CB2 3EJ Cambridge, U. K. 2) Department of Human Behaviour, Ecology, and Culture, MPI for Evolutionary Anthropology, Deutscher Platz 6, 04103 Leipzig, -

Students Spotlight View Mismannered in This Issue
H UMAN BE H AVIOR & EVOLUTION SOCIETY Summer 2008 Newsletter In This Issue View From the President’s Window Spotlight The Student Voice Call for Nominations! Competition Winners Conference News HBES 2008 Japan The next HBES Conference will be held at California Letters From the Editors State University, Fullerton May 27-31, 2009. Announcements Nominations for the HBES Career Awards Job Announcements Darwin 200 in Chile Daniel. G Freedman: Submit your nominations for the HBES Lifetime & 1927-2008 Early Career Contribution Awards. Read more... Resources View Spotlight MisMannered Students From the President’s Window Richard D. Alexander Doug Kenrick The Student Voice | Aaron Blackwell Steve Gangestad Our HBES president is Steve Instead of the typical MisMannered is currently on It is time to nominate Gangestad, Distinguished interview, in this edition, a well-deserved hiatus. I’d a new HBES Student Professor of Psychology we here from Richard like to take this opportunity Representative. Current at the University of New Alexander, winner of the to say a big thank you to student rep Aaron Blackwell Mexico. In this issue, Steve inaugural HBES Lifetime Doug for entertaining us in puts out the call for students continues a discussion on Career Contribution Award. the last few newsletters! interested in this post. patterns of citiations in the Prof. Alexander contiunes Stay tuned for upcoming Also, read field. He his discussion editions of the provides of topics MisMannered the winning some data on included in column. I am abstracts the growth of his HBES sure it will be from this citations of 2008 Keynote a treat! year’s HBES EHB articles. -

Homo Habilis
COMMENT SUSTAINABILITY Citizens and POLICY End the bureaucracy THEATRE Shakespeare’s ENVIRONMENT James Lovelock businesses must track that is holding back science world was steeped in on surprisingly optimistic governments’ progress p.33 in India p.36 practical discovery p.39 form p.41 The foot of the apeman that palaeo ‘handy man’, anthropologists had been Homo habilis. recovering in southern Africa since the 1920s. This, the thinking went, was replaced by the taller, larger-brained Homo erectus from Asia, which spread to Europe and evolved into Nean derthals, which evolved into Homo sapiens. But what lay between the australopiths and H. erectus, the first known human? BETTING ON AFRICA Until the 1960s, H. erectus had been found only in Asia. But when primitive stone-chop LIBRARY PICTURE EVANS MUSEUM/MARY HISTORY NATURAL ping tools were uncovered at Olduvai Gorge in Tanzania, Leakey became convinced that this is where he would find the earliest stone- tool makers, who he assumed would belong to our genus. Maybe, like the australopiths, our human ancestors also originated in Africa. In 1931, Leakey began intensive prospect ing and excavation at Olduvai Gorge, 33 years before he announced the new human species. Now tourists travel to Olduvai on paved roads in air-conditioned buses; in the 1930s in the rainy season, the journey from Nairobi could take weeks. The ravines at Olduvai offered unparalleled access to ancient strata, but field work was no picnic in the park. Water was often scarce. Leakey and his team had to learn to share Olduvai with all of the wild animals that lived there, lions included. -

Great Physicists
Great Physicists Great Physicists The Life and Times of Leading Physicists from Galileo to Hawking William H. Cropper 1 2001 1 Oxford New York Athens Auckland Bangkok Bogota´ Buenos Aires Cape Town Chennai Dar es Salaam Delhi Florence HongKong Istanbul Karachi Kolkata Kuala Lumpur Madrid Melbourne Mexico City Mumbai Nairobi Paris Sao Paulo Shanghai Singapore Taipei Tokyo Toronto Warsaw and associated companies in Berlin Ibadan Copyright ᭧ 2001 by Oxford University Press, Inc. Published by Oxford University Press, Inc. 198 Madison Avenue, New York, New York 10016 Oxford is a registered trademark of Oxford University Press All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of Oxford University Press. Library of Congress Cataloging-in-Publication Data Cropper, William H. Great Physicists: the life and times of leadingphysicists from Galileo to Hawking/ William H. Cropper. p. cm Includes bibliographical references and index. ISBN 0–19–513748–5 1. Physicists—Biography. I. Title. QC15 .C76 2001 530'.092'2—dc21 [B] 2001021611 987654321 Printed in the United States of America on acid-free paper Contents Preface ix Acknowledgments xi I. Mechanics Historical Synopsis 3 1. How the Heavens Go 5 Galileo Galilei 2. A Man Obsessed 18 Isaac Newton II. Thermodynamics Historical Synopsis 41 3. A Tale of Two Revolutions 43 Sadi Carnot 4. On the Dark Side 51 Robert Mayer 5. A Holy Undertaking59 James Joule 6. Unities and a Unifier 71 Hermann Helmholtz 7. The Scientist as Virtuoso 78 William Thomson 8. -
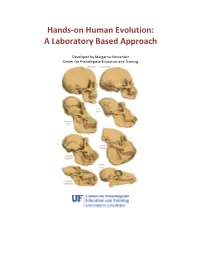
Hands-On Human Evolution: a Laboratory Based Approach
Hands-on Human Evolution: A Laboratory Based Approach Developed by Margarita Hernandez Center for Precollegiate Education and Training Author: Margarita Hernandez Curriculum Team: Julie Bokor, Sven Engling A huge thank you to….. Contents: 4. Author’s note 5. Introduction 6. Tips about the curriculum 8. Lesson Summaries 9. Lesson Sequencing Guide 10. Vocabulary 11. Next Generation Sunshine State Standards- Science 12. Background information 13. Lessons 122. Resources 123. Content Assessment 129. Content Area Expert Evaluation 131. Teacher Feedback Form 134. Student Feedback Form Lesson 1: Hominid Evolution Lab 19. Lesson 1 . Student Lab Pages . Student Lab Key . Human Evolution Phylogeny . Lab Station Numbers . Skeletal Pictures Lesson 2: Chromosomal Comparison Lab 48. Lesson 2 . Student Activity Pages . Student Lab Key Lesson 3: Naledi Jigsaw 77. Lesson 3 Author’s note Introduction Page The validity and importance of the theory of biological evolution runs strong throughout the topic of biology. Evolution serves as a foundation to many biological concepts by tying together the different tenants of biology, like ecology, anatomy, genetics, zoology, and taxonomy. It is for this reason that evolution plays a prominent role in the state and national standards and deserves thorough coverage in a classroom. A prime example of evolution can be seen in our own ancestral history, and this unit provides students with an excellent opportunity to consider the multiple lines of evidence that support hominid evolution. By allowing students the chance to uncover the supporting evidence for evolution themselves, they discover the ways the theory of evolution is supported by multiple sources. It is our hope that the opportunity to handle our ancestors’ bone casts and examine real molecular data, in an inquiry based environment, will pique the interest of students, ultimately leading them to conclude that the evidence they have gathered thoroughly supports the theory of evolution. -

ASEBL Journal
January 2019 Volume 14, Issue 1 ASEBL Journal Association for the Study of EDITOR (Ethical Behavior)•(Evolutionary Biology) in Literature St. Francis College, Brooklyn Heights, N.Y. Gregory F. Tague, Ph.D. ▬ ~ GUEST CO-EDITOR ISSUE ON GREAT APE PERSONHOOD Christine Webb, Ph.D. ~ (To Navigate to Articles, Click on Author’s Last Name) EDITORIAL BOARD — Divya Bhatnagar, Ph.D. FROM THE EDITORS, pg. 2 Kristy Biolsi, Ph.D. ACADEMIC ESSAY Alison Dell, Ph.D. † Shawn Thompson, “Supporting Ape Rights: Tom Dolack, Ph.D Finding the Right Fit Between Science and the Law.” pg. 3 Wendy Galgan, Ph.D. COMMENTS Joe Keener, Ph.D. † Gary L. Shapiro, pg. 25 † Nicolas Delon, pg. 26 Eric Luttrell, Ph.D. † Elise Huchard, pg. 30 † Zipporah Weisberg, pg. 33 Riza Öztürk, Ph.D. † Carlo Alvaro, pg. 36 Eric Platt, Ph.D. † Peter Woodford, pg. 38 † Dustin Hellberg, pg. 41 Anja Müller-Wood, Ph.D. † Jennifer Vonk, pg. 43 † Edwin J.C. van Leeuwen and Lysanne Snijders, pg. 46 SCIENCE CONSULTANT † Leif Cocks, pg. 48 Kathleen A. Nolan, Ph.D. † RESPONSE to Comments by Shawn Thompson, pg. 48 EDITORIAL INTERN Angelica Schell † Contributor Biographies, pg. 54 Although this is an open-access journal where papers and articles are freely disseminated across the internet for personal or academic use, the rights of individual authors as well as those of the journal and its editors are none- theless asserted: no part of the journal can be used for commercial purposes whatsoever without the express written consent of the editor. Cite as: ASEBL Journal ASEBL Journal Copyright©2019 E-ISSN: 1944-401X [email protected] www.asebl.blogspot.com Member, Council of Editors of Learned Journals ASEBL Journal – Volume 14 Issue 1, January 2019 From the Editors Shawn Thompson is the first to admit that he is not a scientist, and his essay does not pretend to be a scientific paper. -

Iciumv LEADERS in ANIMAL BEHAVIOR the Second Generation
UO1BJ3U3D iciumv LEADERS IN ANIMAL BEHAVIOR The Second Generation Edited by Lee C. Drickamer Northern Arizona University Donald A. Dewsbury University of Florida CAMBRIDGE UNIVERSITY PRESS 13 Myths, monkeys, and motherhood: a compromising life SARAH BLAFFER HRDY Definition of an Anthropologist: "(Someone) who studies human nature in all its diversity." Carmelo Lison-Tolosana (1966) Maternal effects (1946-64) From a young age, I was interested in why humans do what they do. With little exposure to science, certainly no inkling that there might be people in the world who studied other animals in order to better understand our species, I decided to become a novelist. Born in Leaders in Animal Behavior: The Setond Generation, ed. L. C. Drickamcr & D. A. Dewsbury. Published by Cambridge University Press. CO Cambridge University Press 2010. 344 Sarah Bluffer Hrdy Texas in 1946, right at the start of the postwar baby boom, I was the third of five children - Speedway. Prevailin four daughters and finally the long-awaited son. My father's father, R. L. Blaffer, had come segregation, and pi to Texas from Hamburg via New Orleans in 1901 at the time oil was discovered at interested in the e1 Spindletop. He recognized that fortunes would be made in the oil business. He married inheritance, female Sarah Campbell from Lampasas, whose father was in that business. I was named for her, the women's moven Sarah Campbell Blaffer II. My mother's father's ancestors, the Hardins, French Huguenots Reared by a suo from Tennessee, arrived earlier, in 1825, before Texas was even a state. -

Laboratory Primate Newsletter
LABORATORY PRIMATE NEWSLETTER Vol. 44, No. 3 July 2005 JUDITH E. SCHRIER, EDITOR JAMES S. HARPER, GORDON J. HANKINSON AND LARRY HULSEBOS, ASSOCIATE EDITORS MORRIS L. POVAR, CONSULTING EDITOR ELVA MATHIESEN, ASSISTANT EDITOR ALLAN M. SCHRIER, FOUNDING EDITOR, 1962-1987 Published Quarterly by the Schrier Research Laboratory Psychology Department, Brown University Providence, Rhode Island ISSN 0023-6861 POLICY STATEMENT The Laboratory Primate Newsletter provides a central source of information about nonhuman primates and re- lated matters to scientists who use these animals in their research and those whose work supports such research. The Newsletter (1) provides information on care and breeding of nonhuman primates for laboratory research, (2) dis- seminates general information and news about the world of primate research (such as announcements of meetings, research projects, sources of information, nomenclature changes), (3) helps meet the special research needs of indi- vidual investigators by publishing requests for research material or for information related to specific research prob- lems, and (4) serves the cause of conservation of nonhuman primates by publishing information on that topic. As a rule, research articles or summaries accepted for the Newsletter have some practical implications or provide general information likely to be of interest to investigators in a variety of areas of primate research. However, special con- sideration will be given to articles containing data on primates not conveniently publishable elsewhere. General descriptions of current research projects on primates will also be welcome. The Newsletter appears quarterly and is intended primarily for persons doing research with nonhuman primates. Back issues may be purchased for $5.00 each. -

A Brief History of Microwave Engineering
A BRIEF HISTORY OF MICROWAVE ENGINEERING S.N. SINHA PROFESSOR DEPT. OF ELECTRONICS & COMPUTER ENGINEERING IIT ROORKEE Multiple Name Symbol Multiple Name Symbol 100 hertz Hz 101 decahertz daHz 10–1 decihertz dHz 102 hectohertz hHz 10–2 centihertz cHz 103 kilohertz kHz 10–3 millihertz mHz 106 megahertz MHz 10–6 microhertz µHz 109 gigahertz GHz 10–9 nanohertz nHz 1012 terahertz THz 10–12 picohertz pHz 1015 petahertz PHz 10–15 femtohertz fHz 1018 exahertz EHz 10–18 attohertz aHz 1021 zettahertz ZHz 10–21 zeptohertz zHz 1024 yottahertz YHz 10–24 yoctohertz yHz • John Napier, born in 1550 • Developed the theory of John Napier logarithms, in order to eliminate the frustration of hand calculations of division, multiplication, squares, etc. • We use logarithms every day in microwaves when we refer to the decibel • The Neper, a unitless quantity for dealing with ratios, is named after John Napier Laurent Cassegrain • Not much is known about Laurent Cassegrain, a Catholic Priest in Chartre, France, who in 1672 reportedly submitted a manuscript on a new type of reflecting telescope that bears his name. • The Cassegrain antenna is an an adaptation of the telescope • Hans Christian Oersted, one of the leading scientists of the Hans Christian Oersted nineteenth century, played a crucial role in understanding electromagnetism • He showed that electricity and magnetism were related phenomena, a finding that laid the foundation for the theory of electromagnetism and for the research that later created such technologies as radio, television and fiber optics • The unit of magnetic field strength was named the Oersted in his honor. -

Lieberman 2001E.Pdf
news and views Another face in our family tree Daniel E. Lieberman The evolutionary history of humans is complex and unresolved. It now looks set to be thrown into further confusion by the discovery of another species and genus, dated to 3.5 million years ago. ntil a few years ago, the evolutionary history of our species was thought to be Ureasonably straightforward. Only three diverse groups of hominins — species more closely related to humans than to chim- panzees — were known, namely Australo- pithecus, Paranthropus and Homo, the genus to which humans belong. Of these, Paran- MUSEUMS OF KENYA NATIONAL thropus and Homo were presumed to have evolved between two and three million years ago1,2 from an early species in the genus Australopithecus, most likely A. afarensis, made famous by the fossil Lucy. But lately, confusion has been sown in the human evolutionary tree. The discovery of three new australopithecine species — A. anamensis3, A. garhi 4 and A. bahrelghazali5, in Kenya, Ethiopia and Chad, respectively — showed that genus to be more diverse and Figure 1 Two fossil skulls from early hominin species. Left, KNM-WT 40000. This newly discovered widespread than had been thought. Then fossil is described by Leakey et al.8. It is judged to represent a new species, Kenyanthropus platyops. there was the finding of another, as yet poorly Right, KNM-ER 1470. This skull was formerly attributed to Homo rudolfensis1, but might best be understood, genus of early hominin, Ardi- reassigned to the genus Kenyanthropus — the two skulls share many similarities, such as the flatness pithecus, which is dated to 4.4 million years of the face and the shape of the brow. -

The Pliocene Hominin Diversity Conundrum: Do More Fossils
SPECIAL FEATURE: PERSPECTIVE The Pliocene hominin diversity conundrum: Do more fossils mean less clarity? SPECIAL FEATURE: PERSPECTIVE Yohannes Haile-Selassiea,b,1, Stephanie M. Melilloc, and Denise F. Sud Edited by Richard G. Klein, Stanford University, Stanford, CA, and approved January 7, 2016 (received for review November 6, 2015) Recent discoveries of multiple middle Pliocene hominins have raised the possibility that early hominins were as speciose as later hominins. However, debates continue to arise around the validity of most of these new taxa, largely based on poor preservation of holotype specimens, small sample size, or the lack of evidence for ecological diversity. A closer look at the currently available fossil evidence from Ethiopia, Kenya, and Chad indicate that Australopithecus afarensis was not the only hominin species during the middle Pliocene, and that there were other species clearly distinguishable from it by their locomotor adaptation and diet. Although there is no doubt that the presence of multiple species during the middle Pliocene opens new windows into our evolutionary past, it also complicates our understanding of early hominin taxonomy and phylogenetic relationships. hominin diversity | Australopithecus | Kenyanthropus | Pliocene | ecological diversity If one looks back over the controversies of human When Australopithecus bahrelghazali was named evolution, they have one element in common: new in 1995 based on an approximately 3.5-Ma partial discoveries, theories, methods came along which “ ” mandible from Chad (12), it was quickly dismissed as no one in the controversy anticipated. The facts – changed, and consequently people were not right a geographic variant of Au. afarensis (13 15). The or wrong in any simple way. -

John Napier's Life Napier's Logarithms KYLIE BRYANT & PAUL SCOTT
KYLIE BRYANT & PAUL SCOTT John Napier’s life Napier’s logarithms John Napier was born in 1550 in the Tower of Napier’s idea in inventing the logarithm was to Merchiston, near Edinburgh, Scotland. His save mathematicians from having to do large father, Archibald Napier, was knighted in 1565 calculations. These days, calculations and was appointed Master of the Mint in 1582. involving large numbers are done using calcu- His mother, Janet Bothwell, was the sister of lators or computers; however before these were the Bishop of Orkney. Napier’s was an impor- invented, large calculations meant that much tant family in Scotland, and had owned the time was taken and mistakes were made. Merchiston estate since 1430. Napier’s logarithm was not defined in terms Napier was educated at St Salvator’s of exponents as our logarithm is today. College, graduating in 1563. He was just 13 Instead Napier thought of his logarithm in years old when his mother died and he was terms of moving particles, distances and veloc- sent to St Andrew’s University where he ities. He considered two lines, AZ of fixed studied for two years. This is where he gained length and A'Z' of infinite length and points X his interest in theology. He did not graduate, and X' starting at A and A' moving to the right however, instead leaving to travel around with the same initial velocity. X' has constant Europe for the next five years. During this velocity and X has a velocity proportional to time he gained knowledge of mathematics and the distance from X to Z.