3. Chemie Von Rotklee …
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Amorpha Fruticosa – a Noxious Invasive Alien Plant in Europe Or a Medicinal Plant Against Metabolic Disease?
fphar-08-00333 June 6, 2017 Time: 18:44 # 1 REVIEW published: 08 June 2017 doi: 10.3389/fphar.2017.00333 Amorpha fruticosa – A Noxious Invasive Alien Plant in Europe or a Medicinal Plant against Metabolic Disease? Ekaterina Kozuharova1, Adam Matkowski2, Dorota Wo´zniak2, Rumiana Simeonova3, Zheko Naychov4, Clemens Malainer5, Andrei Mocan6,7, Seyed M. Nabavi8 and Atanas G. Atanasov9,10,11* 1 Department of Pharmacognosy, Faculty of Pharmacy, Medical University of Sofia, Sofia, Bulgaria, 2 Department of Pharmaceutical Biology with Botanical Garden of Medicinal Plants, Medical University of Wroclaw, Poland, 3 Department of Pharmacology, Pharmacotherapy and Toxicology, Faculty of Pharmacy, Medical University of Sofia, Sofia, Bulgaria, 4 Sofia University St. Kliment Ohridski, Faculty of Medicine, Department of Surgery, Obstetrics and Gynecology, Division of Cardiac Surgery, University Hospital Lozenetz, Sofia, Bulgaria, 5 Independent Researcher, Vienna, Austria, 6 Department of Pharmaceutical Botany, Iuliu Ha¸tieganuUniversity of Medicine and Pharmacy, Cluj-Napoca, Romania, 7 ICHAT and Institute for Life Sciences, University of Agricultural Sciences and Veterinary Medicine, Cluj-Napoca, Romania, 8 Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran, 9 Institute of Genetics and Animal Breeding, Polish Academy of Sciences, Jastrzebiec, Poland, 10 Department of Pharmacognosy, University of Vienna, Vienna, Austria, 11 Department of Vascular Biology and Thrombosis Research, Center for Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria Amorpha fruticosa L. (Fabaceae) is a shrub native to North America which has been Edited by: Kalin Yanbo Zhang, cultivated mainly for its ornamental features, honey plant value and protective properties University of Hong Kong, Hong Kong against soil erosion. -

Flavonoid Glucodiversification with Engineered Sucrose-Active Enzymes Yannick Malbert
Flavonoid glucodiversification with engineered sucrose-active enzymes Yannick Malbert To cite this version: Yannick Malbert. Flavonoid glucodiversification with engineered sucrose-active enzymes. Biotechnol- ogy. INSA de Toulouse, 2014. English. NNT : 2014ISAT0038. tel-01219406 HAL Id: tel-01219406 https://tel.archives-ouvertes.fr/tel-01219406 Submitted on 22 Oct 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Last name: MALBERT First name: Yannick Title: Flavonoid glucodiversification with engineered sucrose-active enzymes Speciality: Ecological, Veterinary, Agronomic Sciences and Bioengineering, Field: Enzymatic and microbial engineering. Year: 2014 Number of pages: 257 Flavonoid glycosides are natural plant secondary metabolites exhibiting many physicochemical and biological properties. Glycosylation usually improves flavonoid solubility but access to flavonoid glycosides is limited by their low production levels in plants. In this thesis work, the focus was placed on the development of new glucodiversification routes of natural flavonoids by taking advantage of protein engineering. Two biochemically and structurally characterized recombinant transglucosylases, the amylosucrase from Neisseria polysaccharea and the α-(1→2) branching sucrase, a truncated form of the dextransucrase from L. Mesenteroides NRRL B-1299, were selected to attempt glucosylation of different flavonoids, synthesize new α-glucoside derivatives with original patterns of glucosylation and hopefully improved their water-solubility. -

Characterization of the Isoflavone Pratensein As a Novel
July 2009 Notes Biol. Pharm. Bull. 32(7) 1289—1294 (2009) 1289 Characterization of the Isoflavone Pratensein as a Novel Transcriptional Up-Regulator of Scavenger Receptor Class B Type I in HepG2 Cells # # Yuan YANG, Wei JIANG, Li WANG, Zhong-Bing ZHANG, Shu-Yi SI,* and Bin HONG* Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College; Beijing 100050, China. Received January 22, 2009; accepted April 9, 2009; published online April 15, 2009 Scavenger receptor class B type I (SR-BI), as well as its human homologue CLA-1, plays an important role in reverse cholesterol transport (RCT) as high density lipoprotein (HDL) receptor. Using a previously developed cell-based screening model for CLA-1 up-regulators, pratensein, was shown to present activity in elevating CLA- 1 transcriptional level. In this study, three other isoflavones including formononetin, genistein and daidzein were also shown to up-regulate CLA-1 transcriptional activity in the cell-based reporter assay. The effects of praten- sein on up-regulating CLA-1 expression were demonstrated at both mRNA and protein levels, and validated by its increasing of 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine perchlorate-labeled (DiI)-HDL uptake in HepG2 cells. Furthermore, the cis-elements responsible for the pratensein up-regulatory effects were mapped to the ؊1055/؊182 bp fragment of CLA-1 promoter in HepG2 cells. These findings might provide a new molecular mechanism by which isoflavones potentially prevent atherosclerosis. Key words pratensein; scavenger receptor class B type I; reverse cholesterol transport; atherosclerosis; cis-element Cardiovascular disease is one of the most common dis- is beneficial in post-menopausal women for bone, cardiovas- eases that damage human health in developed countries and cular risk and hot flashes.9) The hypolipidemic activity of most developing countries, while atherosclerosis is the prin- pratensein has been reported in Triton-WR1339-induced hy- cipal pathogenesis for many critical cardiovascular diseases. -

Relation Structure/Activité De Tanins Bioactifs Contre Les Nématodes
En vue de l'obtention du DOCTORAT DE L'UNIVERSITÉ DE TOULOUSE Délivré par : Institut National Polytechnique de Toulouse (INP Toulouse) Discipline ou spécialité : Pathologie, Toxicologie, Génétique et Nutrition Présentée et soutenue par : Mme JESSICA QUIJADA PINANGO le jeudi 17 décembre 2015 Titre : RELATION STRUCTURE/ACTIVITE DE TANINS BIOACTIFS CONTRE LES NEMATODES GASTROINTESTINAUX (HAEMONCHUS CONTORTUS) PARASITES DES PETITS RUMINANTS Ecole doctorale : Sciences Ecologiques, Vétérinaires, Agronomiques et Bioingénieries (SEVAB) Unité de recherche : Interactions Hôtes - Agents Pathogènes (IHAP) Directeur(s) de Thèse : M. HERVÉ HOSTE Rapporteurs : M. ADIBE LUIZ ABDALLA, UNIVERSIDAD DE SAO PAULO Mme HEIDI ENEMARK, NORWEGIAN VETERINARY INSTITUTE Membre(s) du jury : 1 M. FRANÇOIS SCHELCHER, ECOLE NATIONALE VETERINAIRE DE TOULOUSE, Président 2 M. HERVÉ HOSTE, INRA TOULOUSE, Membre 2 Mme CARINE MARIE-MAGDELAINE, INRA PETIT BOURG, Membre 2 M. SMARO SOTIRAKI, HAO-DEMETER, Membre 2 M. VINCENT NIDERKORN, INRA CLERMONT FERRAND, Membre QUIJADA J. 2015 Cette thèse est dédiée à mes parents, Teresa et Héctor, À mon mari, Rafäel, pour son soutien inconditionnel, son amour illimité, sa patience, sa loyauté, son amitié et surtout sa confidence, À ma grand-mère, Marcolina, car m'ait donné le plus grand et précieux cadeau en ma vie : ma foi en Dieu ma forteresse et mon espoir (Isaïas 41:13). À mes adorés sœurs, belle- sœurs et frère : Yurlin, Indira, Iskay, Olga, Zoraida et Jesus. Merci pour l’amour infini que m’ont toujours été donné, celui qu’a été prolongé par l'amour de mes merveilleux neveux. 1 QUIJADA J. 2015 REMERCIEMENTS Je remercie tout d’abord mon Dieu pour me donner le cadeau de la vie, et la forteresse pour vivre chaque jour. -

IN SILICO ANALYSIS of FUNCTIONAL Snps of ALOX12 GENE and IDENTIFICATION of PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS
Tulasidharan Suja Saranya et al. Int. Res. J. Pharm. 2014, 5 (6) INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 – 8407 Research Article IN SILICO ANALYSIS OF FUNCTIONAL SNPs OF ALOX12 GENE AND IDENTIFICATION OF PHARMACOLOGICALLY SIGNIFICANT FLAVONOIDS AS LIPOXYGENASE INHIBITORS Tulasidharan Suja Saranya, K.S. Silvipriya, Manakadan Asha Asokan* Department of Pharmaceutical Chemistry, Amrita School of Pharmacy, Amrita Viswa Vidyapeetham University, AIMS Health Sciences Campus, Kochi, Kerala, India *Corresponding Author Email: [email protected] Article Received on: 20/04/14 Revised on: 08/05/14 Approved for publication: 22/06/14 DOI: 10.7897/2230-8407.0506103 ABSTRACT Cancer is a disease affecting any part of the body and in comparison with normal cells there is an elevated level of lipoxygenase enzyme in different cancer cells. Thus generation of lipoxygenase enzyme inhibitors have suggested being valuable. Individual variation was identified by the functional effects of Single Nucleotide Polymorphisms (SNPs). 696 SNPs were identified from the ALOX12 gene, out of which 73 were in the coding non-synonymous region, from which 8 were found to be damaging. In silico analysis was performed to determine naturally occurring flavonoids such as isoflavones having the basic 3- phenylchromen-4-one skeleton for the pharmacological activity, like Genistein, Diadzein, Irilone, Orobol and Pseudobaptigenin. O-methylated isoflavones such as Biochanin, Calycosin, Formononetin, Glycitein, Irigenin, 5-O-Methylgenistein, Pratensein, Prunetin, ψ-Tectorigenin, Retusin and Tectorigenine were also used for the study. Other natural products like Aesculetin, a coumarin derivative; flavones such as ajoene and baicalein were also used for the comparative study of these natural compounds along with acteoside and nordihydroguaiaretic acid (antioxidants) and active inhibitors like Diethylcarbamazine, Zileuton and Azelastine as standard for the computational analysis. -

Ginkgo Biloba L., Induces Neuronal Differentiation of Cultured PC12 Cells: Potentiating the Effect of Nerve Growth Factor
Hindawi Publishing Corporation Evidence-Based Complementary and Alternative Medicine Volume 2012, Article ID 278273, 11 pages doi:10.1155/2012/278273 Research Article Isorhamnetin, A Flavonol Aglycone from Ginkgo biloba L., Induces Neuronal Differentiation of Cultured PC12 Cells: Potentiating the Effect of Nerve Growth Factor Sherry L. Xu, Roy C. Y. Choi, Kevin Y. Zhu, Ka-Wing Leung, Ava J. Y. Guo, Dan Bi, Hong Xu, David T. W. Lau, Tina T. X. Dong, and Karl W. K. Tsim Division of Life Science and Center for Chinese Medicine, The Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong Correspondence should be addressed to Karl W. K. Tsim, [email protected] Received 6 March 2012; Accepted 11 April 2012 Academic Editor: Paul Siu-Po Ip Copyright © 2012 Sherry L. Xu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Flavonoids, a group of compounds mainly derived from vegetables and herbal medicines, share a chemical resemblance to estrogen, and indeed some of which have been used as estrogen substitutes. In searching for possible functions of flavonoids, the neuroprotective effect in brain could lead to novel treatment, or prevention, for neurodegenerative diseases. Here, different subclasses of flavonoids were analyzed for its inductive role in neurite outgrowth of cultured PC12 cells. Amongst the tested flavonoids, a flavonol aglycone, isorhamnetin that was isolated mainly from the leaves of Ginkgo biloba L. showed robust induction in the expression of neurofilament, a protein marker for neurite outgrowth, of cultured PC12 cells. -

Phylogenetic Insights on the Isoflavone Profile Variations In
Food Research International 76 (2015) 51–57 Contents lists available at ScienceDirect Food Research International journal homepage: www.elsevier.com/locate/foodres Phylogenetic insights on the isoflavone profile variations in Fabaceae spp.: Assessment through PCA and LDA Tatiana Visnevschi-Necrasov a,b, João C.M. Barreira b,c,⁎,SaraC.Cunhab, Graça Pereira d, Eugénia Nunes a, M. Beatriz P.P. Oliveira b a CIBIO-ICETA, Faculdade de Ciências, Universidade do Porto, R. Padre Armando Quintas 4485-661 Vairão, Portugal b REQUIMTE, Departamento de Ciências Químicas, Faculdade de Farmácia, Universidade do Porto, Rua Jorge Viterbo Ferreira, No. 228, 4050-313, Porto,Portugal c CIMO-ESA, Instituto Politécnico de Bragança, Campus de Santa Apolónia, Apartado 1172, 5301-855 Bragança, Portugal d INRB/IP — INIA — Instituto Nacional de Recursos Biológicos, Caia E São Pedro Estrada Gil Vaz, 7350-228 Elvas, Portugal article info abstract Article history: Legumes (Fabaceae) are important crops, known as sources of food, feed for livestock and raw materials for in- Received 30 September 2014 dustry. Their ability to capture atmospheric nitrogen during symbiotic processes with soil bacteria reduces the Received in revised form 15 November 2014 need for expensive chemical fertilizers, improving soil and water quality. Several Fabaceae species are acknowl- Accepted 20 November 2014 edged for the high levels of secondary metabolites. Isoflavones are among the most well-known examples of Available online 28 November 2014 these compounds, being recognized for their several types of biological activity. Herein, isoflavone profiles were characterized in nine species of four Fabaceae genera (Biserrula, Lotus, Ornithopus and Scorpiurus). Plants Chemical compounds studied in this article: fl Daidzin (PubChem CID: 107971) were harvested in the late ower physiological stage to prevent biased results due to naturally occurring varia- Genistin (PubChem CID: 5281377) tions along the vegetative cycle. -

23 Original Constituents and 147 Metabolites) of Astragali Radix Total Flavonoids and Their Distributions in Rats Using HPLC-DAD-ESI-IT-TOF-Msn
Supplementary Materials Exploring the In Vivo Existence Forms (23 Original Constituents and 147 Metabolites) of Astragali Radix Total Flavonoids and Their Distributions in Rats Using HPLC-DAD-ESI-IT-TOF-MSn Li-Jia Liu, Hong-Fu Li, Feng Xu *, Hong-Yan Wang, Yi-Fan Zhang, Guang-Xue Liu, Ming-Ying Shang, Xuan Wang and Shao-Qing Cai * State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, No. 38 Xueyuan Road, Beijing 100191, China; [email protected] (L.-J.L.); [email protected] (H.-F.L.); [email protected] (H.-Y.W.); [email protected] (Y.-F.Z); [email protected] (G.-X.L.); [email protected] (M.-Y. S); [email protected] (X.W.) * Correspondence: [email protected] (F.X.); [email protected] (S.-Q.C.); Tel.: +86-10-8280-2534 (F.X.); +86-10-8280-1693 (S.-Q.C.) 1. Supplementary Methods 1.1. Detailed Information on the Determination of the Contents of ARTF and its Major Constituents 1.1.1. ARTF Content Determination by HPLC-DAD-ELSD ARTF content determination was performed on a Shimadzu Prominence LC-20A liquid chromatograph system coupled with a low temperature ELSD, consisting of a DGU-20A3 degasser, an LC-20AD binary pump,an SIL-20A autosampler, a CBM-20A communications bus module, an SPD-M20A diode array detector, a CTO-20A column oven, and a low temperature ELSD-LT II detector. The chromatography separations were performed on an Industries Epic C18 column (250mm × 4.6 mm, 5 μm) (New Brunswick, NJ, USA) protected with an Agilent ZORBAX SB C18 guard column (12.5 mm × 4.6 mm, 5 μm) (Santa Clara, CA, USA). -

Ep 3138585 A1
(19) TZZ¥_¥_T (11) EP 3 138 585 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.03.2017 Bulletin 2017/10 A61L 27/20 (2006.01) A61L 27/54 (2006.01) A61L 27/52 (2006.01) (21) Application number: 16191450.2 (22) Date of filing: 13.01.2011 (84) Designated Contracting States: (72) Inventors: AL AT BE BG CH CY CZ DE DK EE ES FI FR GB • Gousse, Cecile GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO 74230 Dingy Saint Clair (FR) PL PT RO RS SE SI SK SM TR • Lebreton, Pierre Designated Extension States: 74000 Annecy (FR) BA ME •Prost,Nicloas 69440 Mornant (FR) (30) Priority: 13.01.2010 US 687048 26.02.2010 US 714377 (74) Representative: Hoffmann Eitle 30.11.2010 US 956542 Patent- und Rechtsanwälte PartmbB Arabellastraße 30 (62) Document number(s) of the earlier application(s) in 81925 München (DE) accordance with Art. 76 EPC: 15178823.9 / 2 959 923 Remarks: 11709184.3 / 2 523 701 This application was filed on 29-09-2016 as a divisional application to the application mentioned (71) Applicant: Allergan Industrie, SAS under INID code 62. 74370 Pringy (FR) (54) STABLE HYDROGEL COMPOSITIONS INCLUDING ADDITIVES (57) The present specification generally relates to hydrogel compositions and methods of treating a soft tissue condition using such hydrogel compositions. EP 3 138 585 A1 Printed by Jouve, 75001 PARIS (FR) EP 3 138 585 A1 Description CROSS REFERENCE 5 [0001] This patent application is a continuation-in-part of U.S. -

Isoflavonoid Biosynthesis 21321.Pdf
Isoflavonoid Biosynthesis https://www.kegg.jp/kegg-bin/highlight_pathway?scale=1.0&map=map00943&keyword=flavonoids Isoflavonoids are biologically active compounds, such as phytoestrogens, produced by pea family plants. While flavonoids (in the narrow sense) have the 2- phenylchromen-4-one backbone, isoflavonoids have the 3-phenylchromen-4-one backbone with no hydroxyl group substitution at position 2. Isoflavonoids are derived from the flavonoid biosynthesis pathway via liquiritigenin or naringenin. (Flavonoid Biosynthesis) -> [1,2] 1) Liquiritigenin -> [1,2,3] 1) flavone synthesis I & II -> 7,4’Dihydroxyflavone OR 2) flavonoid 6-hydroxylase -> 6,7,4’-Trihydroxyflavanone -> 2- hydroxyisoflavone synthase -> 2,6,7,4’-tetrahydroxyisoflavanone -> 6- Hydroxydaidzein -> Glycitein -> isoflavone 7-O-glucosyltransferase -> Glycitein 7-O-glucoside -> isoflavone 7-O-glucoside-6’’-O-malonyltransferase -> Glycitein 7-O-glucoside-6”-O-malonate OR 3) 2-hydroxyisoflavanone synthase -> 2,7,4’Trihydroxyisoflavanone -> [1,2] 1) Feedback Loop 2,7,4’-trihydroxyisoflavanone 4’-O-methyltransferase / isoflavone 4’-O-methyltransferase -> 2,7-Dihydroxy-4’-methoxyisoflavanone -> 2- hydroxyisoflavanone dehydratase -> Formononetin OR 2) 2-hydroxyisoflavone dehydratase -> Daidzein -> [1,2,3,4,5] 1) isoflavone/4’-methoxyisoflavone 2’-hydroxylase -> 2’Hydroxydaidzein -> 2’Hydroxydaidzein reductase -> 2’-Hydroxydihydrodaidzein -> 3,9-Dihydroxypterocarpan -> 3,9-dihydroxypterocarpan 6a-monooxygenase -> 3,6,9- Trihydroxypterocarpan -> [1,2] 1) trihydroxypterocarpan dimethylallyltransferase -

PDF-Document
Supplementary Materials 1 Metabolomics profiling analysis of red clover Table S1. Compound identification of red clover in positive and negative ion modes. MS1 No RT Ion Ion Measur Predicte Ste Le Flow Err MS2 MS3 Identification . min Form Formula ed d m af er ppm m/z m/z [M − 195.050 1 1.43 C6H11O7 195.0499 2.82 Gluconic acid ++ ++ +++ H]- 5 179.03506(C9H7O4); Benzoylcitronensa [M − 295.045 295.0448 ++ 2 4.46 C13H11O8 0.67 133.01451(C4H5O5); [295-179]135.04524(C8H7O2); ure ++ +++ H]- 0 4 + 115.00400(C4H3O4); [M + 355.102 ++ 3 5.42 C16H19O9 355.1024 1.21 chlorogenic acid + +++ H]+ 8 + [433-271]253.04956(C15H9O4); 243.06529(C14H11O4); [M + 433.111 Genistein- ++ 4 6.60 C21H21O10 433.1129 −3.54 271.06067(C15H11O5); 215.07042(C13H11O3); +++ +++ H]+ 4 glucoside + 153.01839(C7H5O4); 149.02335(C8H5O3); [M + 417.117 5 6.71 C21H21O9 417.1180 −2.34 239.07021(C15H11O3) daidzin + ++ +++ H]+ 0 [M + 447.127 calycosin-7-O-β-D- ++ 6 7.20 C22H23O10 447.1286 −1.91 269.08072(C16H13O4) +++ +++ H]+ 7 glucoside + 1 [533-285]270.05258(C15H10O5); Calycosin-7-O-β- [M + 533.127 253.04982(C15H9O4); ++ 7 8.03 C25H25O13 533.1290 −3.54 285.07642(C16H13O5); D-glucoside 4′′-O- +++ +++ H]+ 1 225.05478(C14H9O3); + malonate 137.02333(C7H5O3); [M + 433.112 ++ 8 8.95 C21H21O10 433.1129 −2.11 255.06516(C15H11O4) genistin ++ +++ H]+ 0 + [519-271]253.04958(C15H9O4,); Genistein- 243.06526(C14H11O4); [M + 519.111 433.11377(C21H21O10); glucoside ++ 9 9.31 C24H23O13 519.1133 −3.25 215.07045(C13H11O3); - +++ H]+ 6 271.06073(C15H11O5); malonate + 153.01833(C7H5O4); 149.02333(C8H5O3); -
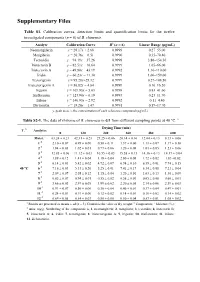
Supplementary Files
Supplementary Files Table S1. Calibration curves, detection limits and quantification limits for the twelve investigated components (n = 6) of B. chinensis. Analyte Calibration Curve R2 (n = 6) Linear Range (μg/mL) Neomangiferin y = 28.17x + 2.68 0.9999 0.27–55.00 Mangiferin y = 20.76x − 6.51 0.9990 0.35–70.40 Tectoridin y = 94.19x − 37.26 0.9998 3.86–154.50 Iristectorin B y = 82.51x − 10.64 0.9999 1.65–66.00 Iristectorin A y = 49.80x − 43.19 0.9992 1.10–110.00 Iridin y = 60.23x − 11.30 0.9999 1.06–159.00 Tectorigenin y = 95.28x+25.32 0.9999 0.27–108.50 Iristectorigenin A y = 56.82x − 4.84 0.9999 0.16–16.20 Irigenin y = 109.93x + 5.03 0.9998 0.83–41.60 Irisflorentin y = 123.90x − 0.19 0.9993 0.23–11.70 Irilone y = 146.93x − 2.92 0.9992 0.11–4.40 Dictomitin y = 18.20x − 1.47 0.9991 0.59–17.70 y, peak area; x, the concentration of each reference compound (μg/mL). Table S2-1. The data of rhizome of B. chinensis in G1 from different sampling points at 40 °C. a Drying Time (min) T. b Analytes 0 120 240 360 480 600 Moist. c 63.24 ± 0.21 42.31 ± 0.23 27.25 ± 0.06 20.14 ± 0.10 12.04 ± 0.13 8.13 ± 0.06 1 d 2.10 ± 0.07 0.95 ± 0.00 0.50 ± 0.11 1.37 ± 0.00 1.11 ± 0.07 1.17 ± 0.10 2 d 1.84 ± 0.03 1.02 ± 0.01 0.77 ± 0.06 1.20 ± 0.08 1.03 ± 0.05 1.23 ± 0.06 3 d 12.85 ± 0.06 11.12 ± 0.03 10.55 ± 0.03 15.58 ± 0.13 14.36 ± 0.13 14.17 ± 0.04 4 d 1.89 ± 0.12 1.41 ± 0.04 1.19 ± 0.04 2.00 ± 0.08 1.72 ± 0.02 1.83 ±0.02 5 d 6.14 ± 0.03 5.42 ± 0.02 4.72 ± 0.07 6.94 ± 0.10 6.59 ± 0.01 7.74 ± 0.15 40 °C 6 d 7.16 ± 0.03 5.33 ± 0.26 5.25 ± 0.01 7.41 ± 0.17 6.34 ±