Study on Environmental Factors of Fluorine in Chagan Lake Catchment, Northeast China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

Annual Report 2019
HAITONG SECURITIES CO., LTD. 海通證券股份有限公司 Annual Report 2019 2019 年度報告 2019 年度報告 Annual Report CONTENTS Section I DEFINITIONS AND MATERIAL RISK WARNINGS 4 Section II COMPANY PROFILE AND KEY FINANCIAL INDICATORS 8 Section III SUMMARY OF THE COMPANY’S BUSINESS 25 Section IV REPORT OF THE BOARD OF DIRECTORS 33 Section V SIGNIFICANT EVENTS 85 Section VI CHANGES IN ORDINARY SHARES AND PARTICULARS ABOUT SHAREHOLDERS 123 Section VII PREFERENCE SHARES 134 Section VIII DIRECTORS, SUPERVISORS, SENIOR MANAGEMENT AND EMPLOYEES 135 Section IX CORPORATE GOVERNANCE 191 Section X CORPORATE BONDS 233 Section XI FINANCIAL REPORT 242 Section XII DOCUMENTS AVAILABLE FOR INSPECTION 243 Section XIII INFORMATION DISCLOSURES OF SECURITIES COMPANY 244 IMPORTANT NOTICE The Board, the Supervisory Committee, Directors, Supervisors and senior management of the Company warrant the truthfulness, accuracy and completeness of contents of this annual report (the “Report”) and that there is no false representation, misleading statement contained herein or material omission from this Report, for which they will assume joint and several liabilities. This Report was considered and approved at the seventh meeting of the seventh session of the Board. All the Directors of the Company attended the Board meeting. None of the Directors or Supervisors has made any objection to this Report. Deloitte Touche Tohmatsu (Deloitte Touche Tohmatsu and Deloitte Touche Tohmatsu Certified Public Accountants LLP (Special General Partnership)) have audited the annual financial reports of the Company prepared in accordance with PRC GAAP and IFRS respectively, and issued a standard and unqualified audit report of the Company. All financial data in this Report are denominated in RMB unless otherwise indicated. -

Annual Report 2017 FINANCIAL HIGHLIGHTS
CONTENT Corporate Information 2 Financial Highlights 3 Chairman’s Statement 4 Management Discussion and Analysis 7 Corporate Governance Report 14 Environmental, Social and Governance Report 22 Directors and Senior Management 37 Directors’ Report 40 Independent Auditor’s Report 49 Consolidated Statement of Profit or Loss 55 Consolidated Statement of Profit or Loss and Other Comprehensive Income 56 Consolidated Statement of Financial Position 57 Consolidated Statement of Changes in Equity 59 Consolidated Cash Flow Statement 60 Notes to the Financial Statements 62 Financial Summary 100 CORPORATE INFORMATION (as at 25 April 2018) BOARD OF DIRECTORS PRINCIPAL PLACE OF BUSINESS IN Executive Directors HONG KONG Mr. Zhao Jinmin (Chairman) Room 6636, 66th Floor Mr. Liu Yingwu The Center 99 Queen’s Road Central Mr. Wang Qingguo Central Mr. Xu Huilin (Chief Executive Officer) Hong Kong Independent Non-Executive Directors PRINCIPAL SHARE REGISTRAR AND Ms. Su Dan TRANSFER OFFICE Mr. Yu Chen Conyers Trust Company (Cayman) Limited Mr. Lau Ying Kit Cricket Square Hutchins Drive, P.O. Box 2681 Grand Cayman KY1-1111 COMPANY SECRETARY Cayman Islands Mr. Lo Wai Kit, ACCA, FCPA, CFA HONG KONG BRANCH SHARE REGISTRAR AUTHORIZED REPRESENTATIVES AND TRANSFER OFFICE Mr. Xu Huilin Tricor Investor Services Limited Mr. Lo Wai Kit Level 22, Hopewell Centre 183 Queen’s Road East MEMBERS OF AUDIT COMMITTEE Hong Kong Mr. Lau Ying Kit (Chairman) PRINCIPAL BANKERS Ms. Su Dan Wing Lung Bank Limited Mr. Yu Chen China Construction Bank Industrial and Commercial Bank of China MEMBERS OF REMUNERATION COMMITTEE Mr. Yu Chen (Chairman) HONG KONG LEGAL ADVISER Mr. Liu Yingwu P. C. -

History and Corporate Structure
THIS WEB PROOF INFORMATION PACK IS IN DRAFT FORM. The information contained in it is incomplete and is subject to change. This Web Proof Information Pack must be read in conjunction with the section headed “Warning” on the cover of this Web Proof Information Pack. HISTORY AND CORPORATE STRUCTURE HISTORY On December 16, 1997, GOC, an Independent Third Party, and CNPC entered into the original production sharing contracts in relation to the Daan and Miao 3 oilfields. GOC is an investment holding company incorporated in the Bahamas owned by a number of Australian shareholders, some of whom have experience in the oil industry, and its only oil/gas asset is its interest in the three production sharing contracts. On September 25, 1998, GOC and CNPC entered into the original production sharing contract in relation to the Moliqing oilfield. Pursuant to an assignment agreement entered into between CNPC and its subsidiary, PetroChina, on December 23, 1999, CNPC assigned to PetroChina most of its commercial and operational rights and obligations under the three production sharing contracts. GOC became the foreign contractor and the operator of the three oilfields pursuant to these production sharing contracts. Under the assignment agreement, CNPC retained certain specific rights in relation to the three production sharing contracts in the following aspects: (i) any amendment to material provisions of the production sharing contracts which requires the approval by the MOFCOM shall be reported to CNPC and be referred by CNPC to the original examination -

2020 Annual Report.Pdf
HAITONG SECURITIES CO., LTD. 海通證券股份有限公司 Annual Report 2020 年度報告2020 年度報告 Annual Report 2020 CONTENTS Section I DEFINITIONS AND MATERIAL RISK WARNINGS 3 Section II COMPANY PROFILE AND KEY FINANCIAL INDICATORS 7 Section III SUMMARY OF THE COMPANY’S BUSINESS 25 Section IV REPORT OF THE BOARD OF DIRECTORS 33 Section V SIGNIFICANT EVENTS 85 Section VI CHANGES IN ORDINARY SHARES AND PARTICULARS ABOUT SHAREHOLDERS 123 Section VII PREFERENCE SHARES 136 Section VIII DIRECTORS, SUPERVISORS, SENIOR MANAGEMENT AND EMPLOYEES 137 Section IX CORPORATE GOVERNANCE 191 Section X CORPORATE BONDS 229 Section XI FINANCIAL REPORT 240 Section XII DOCUMENTS AVAILABLE FOR INSPECTION 241 Section XIII INFORMATION DISCLOSURES OF SECURITIES COMPANY 242 2 HAITONG SECURITIES CO., LTD. | Annual Report 2020 (H Share) IMPORTANT NOTICE The Board, the Supervisory Committee, Directors, Supervisors and senior management of the Company warrant the truthfulness, accuracy and completeness of contents of this annual report (the “Report”) and that there is no false representation, misleading statement contained herein or material omission from this Report, for which they will assume joint and several liabilities. This Report was considered and approved at the 15th meeting of the seventh session of the Board. All the Directors of the Company attended the Board meeting. None of the Directors or Supervisors has made any objection to this Report. PricewaterhouseCoopers Zhong Tian LLP (Special General Partnership) and PricewaterhouseCoopers have audited the annual financial reports of the Company prepared in accordance with PRC GAAP and IFRS respectively, and issued a standard and unqualified audit report of the Company. All financial data in this Report are denominated in RMB unless otherwise indicated. -

21 May 2019 Should Be Worn If One Has to Go Out
a man died in a field in central Vietnam. After that, the examinations suggested that his death was because of heat stroke. Ha Tinh Province was recorded the nation’s highest ever temperature in April which hit 43.4 degrees Celsius. WEEKLY UPDATE ON According to the National Hydro ASEAN PLUS THREE Meteorological Forecasting Center, the FOOD SECURITY heat wave was the result of developing low- pressure from the west. The Emergency RELATED INFORMATION Department at the Loc Ha District General Hospital warned that people should not going out between 11:00 a.m. and 3:30 No. 118 p.m. (local time) during hot days to prevent heat strokes. Full-body clothing and masks 15 - 21 May 2019 should be worn if one has to go out. Moreover, several places across Vietnam are facing with hotter summer than normal. Big cities like Hanoi and Saigon have been Thailand hit with extremely high levels of UV rays and sweltering temperatures. Drought has remained amid rainy- season. Although Thailand has currently Source: Vnexpress (2019, May 21). Heat stroke kills man in central Vietnam. entered the rainy-season since 20 May 2019, Nakhon Ratchasima still has faced China up with drought. Drought attacked southwest China in According to the Regional Irrigation Office Yunnan Province. Drought affected 8 Nakhon Ratchasima, several dams and 309,000 people to hardly access drinking reservoirs contained the water under their water. Meanwhile, 93,000 domestic capacity such as Lam Takong Dam animals have been facing a water contained at 55.48 percent of its capacity shortage. In response to the severe and Lum Phra Ploeng Reservoir contained drought, government authorities have sent only 17.96 percent of its capacity. -
Use Style: Paper Title
2018 International Conference on Sensor Network and Computer Engineering (ICSNCE 2018) Earthquake Damage Predicting System of Songyuan Based on GIS Su Zhenjiang Zhang Dian Institute of Disaster prevention Institute of Disaster prevention The GIS Association of Institution of Disaster Prevention The GIS Association of Institution of Disaster Prevention Beijing, China Beijing, China e-mail: [email protected] e-mail: [email protected] Huang Meng* Wang Zhe Institute of Disaster prevention Institute of Disaster prevention The GIS Association of Institution of Disaster Prevention The GIS Association of Institution of Disaster Prevention Beijing, China Beijing, China e-mail: [email protected] e-mail: [email protected] Xie Shaohui Institute of Disaster prevention The GIS Association of Institution of Disaster Prevention Beijing, China e-mail: [email protected] Abstract—With the rapid development of cities, the research which made a rational analysis of the influence of urban pipe on urban seismic damage prediction should have a network connectivity and damage on pipe network, but it breakthrough based on the modern technology. The paper didn’t comprehensively discuss the influence of pipeline takes Songyuan of Jilin Province as the research object, and network on urban buildings. In 2012, Zhao Qi, a director of creates a spatial database by field data and Baidu maps. We the Structural Engineering and Disaster Prevention Research simulate the earthquake by using the seismic intensity Institute of Tongji University, made a Study on Application algorithm of the point source and the line source, use the of High Resolution Remote Sensing Images in Rapid spatial analysis method of PHP+GIS to simulate the influence Prediction of Earthquake Disaster in Urban Area. -
Printmgr File
DIRECTORS, SUPERVISORS AND SENIOR MANAGEMENT DIRECTORS The board of directors of the Bank consists of thirteen directors, of whom three are executive directors, five are non-executive directors and five are independent non-executive directors. Directors are appointed by the Shareholders for a term of three years, and may be appointed for consecutive terms. The term of independent non-executive directors shall not exceed a cumulative period of six years. The following table sets out information regarding the Bank’s directors, all of whom satisfy the qualification requirements under relevant PRC laws and regulations for their respective positions. Relationship with other Date of joining directors, the Bank (or the Date of supervisors Bank’s appointment as and senior Name Age Position predecessor) director Responsibilities management Mr. Gao Bing ( ) .......... 48 Chairman, December December Responsible for None Executive Director, 2004 2008 overall operations Secretary of the and strategic Party committee management, make material decisions and develop the business strategy Mr. Liang Xiangmin ( ) ........ 50 Executive Director, August April 2016 Responsible for the None Vice-president and 1985 business operations Chief Operating and management of Officer branches outside the Jiutai region, participate in making material business decisions and manage certain business departments and offices Mr. Yuan Chunyu ( ) ........ 44 Executive Director, November December Responsible for the None Secretary to the 2010 2012 management of the Board and -

Annual Development Report on China's Trademark Strategy 2013
Annual Development Report on China's Trademark Strategy 2013 TRADEMARK OFFICE/TRADEMARK REVIEW AND ADJUDICATION BOARD OF STATE ADMINISTRATION FOR INDUSTRY AND COMMERCE PEOPLE’S REPUBLIC OF CHINA China Industry & Commerce Press Preface Preface 2013 was a crucial year for comprehensively implementing the conclusions of the 18th CPC National Congress and the second & third plenary session of the 18th CPC Central Committee. Facing the new situation and task of thoroughly reforming and duty transformation, as well as the opportunities and challenges brought by the revised Trademark Law, Trademark staff in AICs at all levels followed the arrangement of SAIC and got new achievements by carrying out trademark strategy and taking innovation on trademark practice, theory and mechanism. ——Trademark examination and review achieved great progress. In 2013, trademark applications increased to 1.8815 million, with a year-on-year growth of 14.15%, reaching a new record in the history and keeping the highest a mount of the world for consecutive 12 years. Under the pressure of trademark examination, Trademark Office and TRAB of SAIC faced the difficuties positively, and made great efforts on soloving problems. Trademark Office and TRAB of SAIC optimized the examination procedure, properly allocated examiners, implemented the mechanism of performance incentive, and carried out the “double-points” management. As a result, the Office examined 1.4246 million trademark applications, 16.09% more than last year. The examination period was maintained within 10 months, and opposition period was shortened to 12 months, which laid a firm foundation for performing the statutory time limit. —— Implementing trademark strategy with a shift to effective use and protection of trademark by law. -
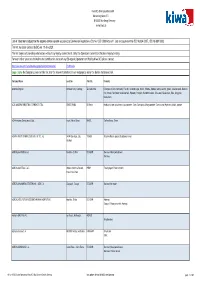
Operators Subject to the EU Controll System with Ecert 10.01.2020
Kiwa BCS Öko-Garantie GmbH Marientorgraben 3-5 D-90402 Nürnberg, Germany www.kiwa.de List of Operators subject to the organic control system according to Commission Regulations (EC) No 1235/2008 Article 11 (3e) and equivalent to (EC) No 834/2007, (EC) No 889/2008. This list has been updated by BCS on 10-Jan-2020. This list targets at providing information without any legally commitment. Only the Operators' current Certificate is legally binding. For any further questions related to the certification status of any EU-organic Operator certified by Kiwa BCS please contact https://www.kiwa.com/de/de/aktuelle-angelegenheiten/zertifikatssuche/ [email protected] copy / paste the Company's name of this list into the relevant datafield of our webpage by using the before mentioned link. Company Name Location Country Products Absolute Organix Birnham Park, Gauteng ZA Suedafrika Chickpeas (Cicer arietinum), Flax oil, Coconut sugar, Millet, Polenta, Quinoa, white, Lentils, green, Sesame seeds, Basmati rice, brown, Sunflower seeds/kernels, Popcorn, Pumpkin (Cucurbit) seeds, Chia seed, Coconut oil, Rice, long grain, Maize/corn, AÇAÍ AMAZONAS INDUSTRIA E COMERCIO LTDA. OBIDOS, PARA BR Brasil Products as per attachment, Acai powder, Camu Camu pulp, Mango powder, Camu-camu (Myrciaria dubia), powder AGA Armazéns Gerais Araxá Ltda. Araxá, Minas Gerais BRAZIL Coffee Beans, Green AGRANA FRUIT İSTANBUL GIDA SAN. VE TİC. A.Ş. 34394 Esentepe, Şişli, TURKEY Black mulberry puree; Blackberry Puree İstanbul AGRÍCOLA AURCRISA C.A. Machala - El Oro ECUADOR Bananas (Musa paradisiaca) Bananas AGRICOLA AUSTRAL S.A.C. Urbano (frente a Senati), PERU Fresh ginger; Fresh turmeric Piura-Piura-Piura AGRÍCOLA BANANERA ECUATORIANA - AGBE S.A. -

Information Memorandum for the Listing of Rui Long Yi Da Commerce Group Limited
Dutch Caribbean Securities Exchange, Listing Advisor 荷兰加勒⽐证券交易所上市保荐机构 Information Memorandum For the Listing of Rui Long Yi Da Commerce Group Limited Incorporated under the laws of BVI Business Companies Act, 2004 (No. 16 of 2004) on November 15th, 2017 with BVI Company Number 1961060 www.chinacccg.com 0 Table of Contents 1. Summary of Certain Defined Terms ....................................................................................................... 2 2. Important Information and Notices ......................................................................................................... 3 3. Issuer and the List of Institutions Related to the Listing ........................................................................ 6 4. Company Overview ................................................................................................................................ 7 4.1 Business Structure ................................................................................................................................................ 7 4.2 About the Company ............................................................................................................................................. 8 4.3 Company’s Operation .......................................................................................................................................... 8 5. Terms of the Issuance and Investment Overview ................................................................................. 10 6. Business Overview ............................................................................................................................... -

Attachment 1
RH PETROGAS LIMITED (Company Registration Number: 198701138Z) ANNOUNCEMENT PROPOSED DISPOSAL OF MASTIQUE INVESTMENTS LIMITED 1. INTRODUCTION 1.1 Proposed Disposal The Board of Directors (the “Board”) of RH Petrogas Limited (the “Company”, together with its subsidiaries, the “RHP Group”) refers to the Company’s announcement dated 8 March 2019 (Announcement Reference SG190308OTHR3Q3I) (the “8 March 2019 Announcement”). The Board is pleased to announce that RH Petrogas Investments Pte. Ltd. (“RHPI”), a wholly-owned subsidiary of the Company, has today entered into a share purchase agreement (the “Agreement”) with 吉林成阳能源开发技术服务有限公司(Jilin Chengyang Energy Development Technology Service Co., Ltd) (the “Purchaser”), pursuant to which RHPI has agreed to sell and the Purchaser has agreed to purchase all of the issued shares in Mastique Investments Limited (“Mastique” and the shares, the “Sale Shares”) (the “Proposed Disposal”). 1.2 Closing Completion of the Proposed Disposal (“Closing”) is subject to and conditional upon certain conditions precedent (“Conditions”) as set out in paragraph 3.4 below. Following Closing, RHPI will no longer hold any interest in Mastique and its only subsidiary (the “Mastique Group”), and the Mastique Group will cease to be a member of the RHP Group. 2. INFORMATION ON THE PARTIES 2.1 Information on RHPI RHPI was incorporated on 22 July 2002 in Singapore and is an investment holding vehicle. As at the date of this Announcement, RHPI has an issued and paid-up capital of S$340,766 comprising 340,766 ordinary shares and the Company owns the entire issued share capital of RHPI. 2.2 Information on Mastique Mastique was incorporated on 2 July 2008 in the British Virgin Islands.