What Is Synthetic Genomics Anyway?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Transcriptional Landscape of a Rewritten Bacterial Genome Reveals Control Elements and Genome Design Principles ✉ ✉ Mariëlle J
ARTICLE https://doi.org/10.1038/s41467-021-23362-y OPEN The transcriptional landscape of a rewritten bacterial genome reveals control elements and genome design principles ✉ ✉ Mariëlle J. F. M. van Kooten 1 , Clio A. Scheidegger1, Matthias Christen1 & Beat Christen 1 Sequence rewriting enables low-cost genome synthesis and the design of biological systems with orthogonal genetic codes. The error-free, robust rewriting of nucleotide sequences can 1234567890():,; be achieved with a complete annotation of gene regulatory elements. Here, we compare transcription in Caulobacter crescentus to transcription from plasmid-borne segments of the synthesized genome of C. ethensis 2.0. This rewritten derivative contains an extensive amount of supposedly neutral mutations, including 123’562 synonymous codon changes. The tran- scriptional landscape refines 60 promoter annotations, exposes 18 termination elements and links extensive transcription throughout the synthesized genome to the unintentional intro- duction of sigma factor binding motifs. We reveal translational regulation for 20 CDS and uncover an essential translational regulatory element for the expression of ribosomal protein RplS. The annotation of gene regulatory elements allowed us to formulate design principles that improve design schemes for synthesized DNA, en route to a bright future of iteration- free programming of biological systems. 1 Institute of Molecular Systems Biology, Department of Biology, Eidgenössische Technische Hochschule Zürich, Zürich, Switzerland. ✉ email: [email protected]; [email protected] NATURE COMMUNICATIONS | (2021) 12:3053 | https://doi.org/10.1038/s41467-021-23362-y | www.nature.com/naturecommunications 1 ARTICLE NATURE COMMUNICATIONS | https://doi.org/10.1038/s41467-021-23362-y e can program biological systems with DNA that is In previous work, we synthesized and assembled the rewritten Wbased on native nucleotide sequences. -

Horizontal Gene Flow Into Geobacillus Is Constrained by the Chromosomal Organization of Growth and Sporulation
bioRxiv preprint doi: https://doi.org/10.1101/381442; this version posted August 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Horizontal gene flow into Geobacillus is constrained by the chromosomal organization of growth and sporulation Alexander Esin1,2, Tom Ellis3,4, Tobias Warnecke1,2* 1Molecular Systems Group, Medical Research Council London Institute of Medical Sciences, London, United Kingdom 2Institute of Clinical Sciences, Faculty of Medicine, Imperial College London, London, United Kingdom 3Imperial College Centre for Synthetic Biology, Imperial College London, London, United Kingdom 4Department of Bioengineering, Imperial College London, London, United Kingdom *corresponding author ([email protected]) 1 bioRxiv preprint doi: https://doi.org/10.1101/381442; this version posted August 2, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. Abstract Horizontal gene transfer (HGT) in bacteria occurs in the context of adaptive genome architecture. As a consequence, some chromosomal neighbourhoods are likely more permissive to HGT than others. Here, we investigate the chromosomal topology of horizontal gene flow into a clade of Bacillaceae that includes Geobacillus spp. Reconstructing HGT patterns using a phylogenetic approach coupled to model-based reconciliation, we discover three large contiguous chromosomal zones of HGT enrichment. -

Section 4. Guidance Document on Horizontal Gene Transfer Between Bacteria
306 - PART 2. DOCUMENTS ON MICRO-ORGANISMS Section 4. Guidance document on horizontal gene transfer between bacteria 1. Introduction Horizontal gene transfer (HGT) 1 refers to the stable transfer of genetic material from one organism to another without reproduction. The significance of horizontal gene transfer was first recognised when evidence was found for ‘infectious heredity’ of multiple antibiotic resistance to pathogens (Watanabe, 1963). The assumed importance of HGT has changed several times (Doolittle et al., 2003) but there is general agreement now that HGT is a major, if not the dominant, force in bacterial evolution. Massive gene exchanges in completely sequenced genomes were discovered by deviant composition, anomalous phylogenetic distribution, great similarity of genes from distantly related species, and incongruent phylogenetic trees (Ochman et al., 2000; Koonin et al., 2001; Jain et al., 2002; Doolittle et al., 2003; Kurland et al., 2003; Philippe and Douady, 2003). There is also much evidence now for HGT by mobile genetic elements (MGEs) being an ongoing process that plays a primary role in the ecological adaptation of prokaryotes. Well documented is the example of the dissemination of antibiotic resistance genes by HGT that allowed bacterial populations to rapidly adapt to a strong selective pressure by agronomically and medically used antibiotics (Tschäpe, 1994; Witte, 1998; Mazel and Davies, 1999). MGEs shape bacterial genomes, promote intra-species variability and distribute genes between distantly related bacterial genera. Horizontal gene transfer (HGT) between bacteria is driven by three major processes: transformation (the uptake of free DNA), transduction (gene transfer mediated by bacteriophages) and conjugation (gene transfer by means of plasmids or conjugative and integrated elements). -
Bacterial Genetics a Tiny Alternative
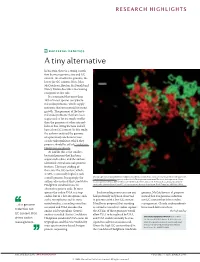
RESEARCH HIGHLIGHTS BACTERIAL GENETICS A tiny alternative In bacteria, there is a strong correla- tion between genome size and GC content: the smaller the genome, the lower the GC content. Now, John McCutcheon, Bradon McDonald and Nancy Moran describe a fascinating exception to this rule. It is estimated that more than 10% of insect species carry bacte- rial endosymbionts, which supply nutrients that are essential for insect growth. The genomes of the bacte- rial endosymbionts that have been sequenced so far are much smaller than the genomes of other intracel- lular or free-living bacteria and all have a low GC content. In this study, the authors analysed the genome of a previously uncharacterized cicada endosymbiont, which they propose should be called Candidatus Hodgkinia cicadicola. At 144 kb, this is the smallest bacterial genome that has been sequenced to date, and the authors identified several unusual genomic features. The most striking of these was the GC content, which, at 58%, is unusually high for such Micrograph showing Candidatus Hodgkinia cicadicola (red) in close association with another endosymbiont, a small genome. Intriguingly, the Candidatus Sulcia muelleri (green) in the cicada Diceroprocta semicincta. The scale bar represents 10 µm. authors also noticed that Candidatus Image reproduced from McCutcheon, J. P., McDonald, B. R. & Moran, N. P. Origin of an alternative genetic Hodgkinia cicadicola uses an code in the extremely small and GC-rich genome of a bacterial symbiont. PLoS Genet. 5, e1000565 (2009). alternative genetic code. In most species the codon UGA is a stop Such recoding events are rare and genome, McCutcheon et al. -

Synthetic Biology Projects in Vitro
Downloaded from genome.cshlp.org on September 25, 2021 - Published by Cold Spring Harbor Laboratory Press Review Synthetic biology projects in vitro Anthony C. Forster1,3 and George M. Church2,3 1Department of Pharmacology and Vanderbilt Institute of Chemical Biology, Vanderbilt University Medical Center, Nashville, Tennessee 37232, USA; 2Department of Genetics, Harvard Medical School, Boston, Massachusetts 02115, USA Advances in the in vitro synthesis and evolution of DNA, RNA, and polypeptides are accelerating the construction of biopolymers, pathways, and organisms with novel functions. Known functions are being integrated and debugged with the aim of synthesizing life-like systems. The goals are knowledge, tools, smart materials, and therapies. Synthetic biology projects (SBPs) an action plan to regulate world suppliers of DNA synthesizers, DNA precursors, and oligos (Church 2004). Ethical and safety The basic elements of chemistry and biology are few, but the issues have been, and must continue to be, regulated (Cho et al. synthetic combinations are unlimited and awe inspiring. The 1999). first international conference on synthetic biology charted its Several groups have proposed to create bacteria with chro- goals as understanding and utilizing life’s diverse solutions to mosomes synthesized entirely from synthetic oligos. This might process information, materials, and energy (Silver and Way 2004) be done stepwise (Posfai et al. 2006) or by inactivating the en- (http://syntheticbiology.org). As a bonus, genetic systems are dogenous bacterial chromosome and then somehow transform- biocompatible, renewable, and can be optimized by Darwinian ing and rebooting the bacterium with an entire in vitro- selections. SBPs entail the complex manipulation of replicating synthesized genome. -

Assessment of the Bimodality in the Distribution of Bacterial Genome Sizes
The ISME Journal (2017) 11, 821–824 © 2017 International Society for Microbial Ecology All rights reserved 1751-7362/17 www.nature.com/ismej SHORT COMMUNICATION Assessment of the bimodality in the distribution of bacterial genome sizes Hyun S Gweon, Mark J Bailey and Daniel S Read Centre for Ecology & Hydrology, Wallingford, UK Bacterial genome sizes have previously been shown to exhibit a bimodal distribution. This phenomenon has prompted discussion regarding the evolutionary forces driving genome size in bacteria and its ecological significance. We investigated the level of inherent redundancy in the public database and the effect it has on the shape of the apparent bimodal distribution. Our study reveals that there is a significant bias in the genome sequencing efforts towards a certain group of species, and that correcting the bias using species nomenclature and clustering of the 16S rRNA gene, results in a unimodal rather than the previously published bimodal distribution. The true genome size distribution and its wider ecological implications will soon emerge as we are currently witnessing rapid growth in the number of sequenced genomes from diverse environmental niches across a range of habitats at an unprecedented rate. The ISME Journal (2017) 11, 821–824; doi:10.1038/ismej.2016.142; published online 11 November 2016 Significant progress has been made in understanding Wolf in 2008, where it was reported that bacterial interactions between ecology and genome evolution genome sizes show a bimodal distribution (Koonin in prokaryotes. -

Mechanisms Of, and Barriers To, Horizontal Gene Transfer Between Bacteria
FOCUS ON HORIZONTAL GENE TRANSFER MECHANISMS OF, AND BARRIERS TO, HORIZONTAL GENE TRANSFER BETWEEN BACTERIA Christopher M. Thomas* and Kaare M. Nielsen‡ Abstract | Bacteria evolve rapidly not only by mutation and rapid multiplication, but also by transfer of DNA, which can result in strains with beneficial mutations from more than one parent. Transformation involves the release of naked DNA followed by uptake and recombination. Homologous recombination and DNA-repair processes normally limit this to DNA from similar bacteria. However, if a gene moves onto a broad-host-range plasmid it might be able to spread without the need for recombination. There are barriers to both these processes but they reduce, rather than prevent, gene acquisition. The first evidence that horizontal gene transfer (HGT) informational genes of the central cellular machinery could occur was the recognition that virulence deter- such as DNA replication, transcription or translation minants could be transferred between pneumococci in tend not to spread rapidly, even if they confer anti biotic infected mice, a phenomenon that was later shown to resistance, compared for example to single-function- be mediated by the uptake of the genetic material DNA resistance determinants such as β-lactamases or in a process called transformation1. The subsequent aminoglycoside-modifying enzymes. However, the identification of gene transfer mediated by both plas- nature of the transfer mechanism can also determine the mids and viruses and the recognition of transposable organisms and genes that are most often involved. The elements provided the stepping stones to our current purpose of this review is to describe some of the mecha- picture of gene flux and the importance of mobile nisms that lead to horizontal gene acquisitions with a genetic elements2. -

PA00XCSB.Pdf
Leidos Proprietary 1. EXECUTIVE SUMMARY A summary of efforts for the planned, ongoing, and completed projects for the Malaria Vaccine Development Program (MVDP) contract for this reporting period are herein detailed. A compiled Gantt chart including activities associated with each of the projects has been created and included as an attachment to this report. Ongoing projects that will continue through FY2019 include two vaccine development projects, the CSP vaccine development project (CSP Vaccine) and liver stage vaccine development project (Liver Stage Vaccine), as well as the clinical study with RH5 (RH5.1 Clinical Study), the latter to assess long-term immunogenicity in RH5.1/AS01 vaccinees. Of note is that while both the CSP and the liver stage vaccine development projects were initiated as epitope-based projects, these have since been realigned to target whole proteins; therefore, the project names have also been realigned to remove “epitope-based.” Expansion of work on the RCR complex into a vaccine development project (RCR Complex) occurred in early FY2019 and this project will continue until the end date of the contract. Lastly, a new project, the RH5.1 human monoclonal antibody identification and development project (RH5.1 Human mAb), was initiated in early FY2019 and will continue until the end date of the contract. Two projects were completed in FY2019, the blood stage epitope-based vaccine development project and the PD1 blockade inhibitor project (PD1 Block Inh). Leidos continues to seek collaborators for information exchange under NDA, reagent exchange under MTA, and collaboration under CRADA, to expand our body of knowledge and access to reagents with minimal cost to the program. -

Generating a Synthetic Genome by Whole Genome Assembly: X174 Bacteriophage from Synthetic Oligonucleotides
Generating a synthetic genome by whole genome assembly: X174 bacteriophage from synthetic oligonucleotides Hamilton O. Smith, Clyde A. Hutchison III†, Cynthia Pfannkoch, and J. Craig Venter‡ Institute for Biological Energy Alternatives, 1901 Research Boulevard, Suite 600, Rockville, MD 20850 Contributed by J. Craig Venter, November 3, 2003 We have improved upon the methodology and dramatically short- truncated species. Although such oligonucleotides are highly ened the time required for accurate assembly of 5- to 6-kb seg- useful as primers for PCR amplification and DNA sequencing, ments of DNA from synthetic oligonucleotides. As a test of this only small (a few hundred base pairs) synthetic genes can methodology, we have established conditions for the rapid (14- generally be accurately and directly synthesized without multiple day) assembly of the complete infectious genome of bacteriophage repair͞selection steps. For example, the recent report (9) of the X174 (5,386 bp) from a single pool of chemically synthesized assembly of a partially active poliovirus from cloned synthetic oligonucleotides. The procedure involves three key steps: (i) gel segments of DNA from which polio genomic RNA (7,440 bases) purification of pooled oligonucleotides to reduce contamination could be transcribed was quite complex and took many months with molecules of incorrect chain length, (ii) ligation of the oligo- to accomplish. First, segments 400–600 bp long were individually nucleotides under stringent annealing conditions (55°C) to select assembled and cloned, and 5–15 isolates of each were sequenced against annealing of molecules with incorrect sequences, and (iii) to find one that was correct or readily correctable by oligonu- assembly of ligation products into full-length genomes by poly- cleotide mutagenesis. -

Synthetic Genomics and Synthetic Biology Applications Between Hopes and Concerns
Send Orders of Reprints at [email protected] Current Genomics, 2013, 14, 11-24 11 Synthetic Genomics and Synthetic Biology Applications Between Hopes and Concerns 1,2, 1 1 1 Harald König *, Daniel Frank , Reinhard Heil and Christopher Coenen 1Institute for Technology Assessment and Systems Analysis (ITAS); 2Institute of Toxicology and Genetics (ITG), Karlsruhe Institute of Technology, PO box 3640, 76021 Karlsruhe, Germany Abstract: New organisms and biological systems designed to satisfy human needs are among the aims of synthetic ge- nomics and synthetic biology. Synthetic biology seeks to model and construct biological components, functions and or- ganisms that do not exist in nature or to redesign existing biological systems to perform new functions. Synthetic genom- ics, on the other hand, encompasses technologies for the generation of chemically-synthesized whole genomes or larger parts of genomes, allowing to simultaneously engineer a myriad of changes to the genetic material of organisms. Engi- neering complex functions or new organisms in synthetic biology are thus progressively becoming dependent on and con- verging with synthetic genomics. While applications from both areas have been predicted to offer great benefits by mak- ing possible new drugs, renewable chemicals or clean energy, they have also given rise to concerns about new safety, en- vironmental and socio-economic risks – stirring an increasingly polarizing debate. Here we intend to provide an overview on recent progress in biomedical and biotechnological applications of synthetic genomics and synthetic biology as well as on arguments and evidence related to their possible benefits, risks and governance implications. Received on: May 22, 2012- Revised on: October 11, 2012- Accepted on: October 12, 2012 Keywords: Applications, Benefits, Biofuels, Biomedicine, Environment, Risks, Synthetic genomics, Synthetic biology. -

Commissioned Papers Synthetic Genomics: Risks and Benefits For
Commissioned Papers Synthetic Genomics: Risks and Benefits for Science and Society This volume of papers accompanies the report Synthetic Genomics: Options for Governance Synthetic Genomics: Risks and Benefits for Science and Society (this page blank) Synthetic Genomics: Risks and Benefits for Science and Society COMMISSIONED PAPERS TABLE OF CONTENTS Robert Jones Sequence Screening……………………………………………………………...……1-16 Yogesh Sanghvi A Roadmap to the Assembly of Synthetic DNA from Raw Materials……..………….17-33 Ralph S. Baric Synthetic Viral Genomics…………………………………………………………….35-81 Marc S. Collett Impact of Synthetic Genomics on the Threat of Bioterrorism with Viral Agents..………………………………………………………………………..83-103 Diane O. Fleming Risk Assessment of Synthetic Genomics: A Biosafety and Biosecurity Perspective………………………...………………………………….105-164 Franco Furger From Genetically Modified Organisms to Synthetic Biology: Legislation in the European Union, in Six Member Countries, and in Switzerland…………………………………………………………..……..165-184 Synthetic Genomics: Risks and Benefits for Science and Society (this page blank) Synthetic Genomics: Risks and Benefits for Science and Society The following papers were commissioned for the project Synthetic Genomics: Risks and Benefits for Science and Society. These papers formed the basis of many discussions at project workshops and at a large invitational meeting. The information elicited from these meetings, and from the commissioned papers themselves, formed the basis of our report Synthetic Genomics: Options for Governance (http://dspace.mit.edu/handle/1721.1/39141). The views and opinions expressed in these commissioned papers are those of the authors of the papers and not necessarily those of the authors of the report, or of the institutions at which the authors work. Citation: Working Papers for Synthetic Genomics: Risks and Benefits for Science and Society. -

Xenobiology: a New Form of Life As the Ultimate Biosafety Tool Markus Schmidt* Organisation for International Dialogue and Conflict Management, Kaiserstr
Review article DOI 10.1002/bies.200900147 Xenobiology: A new form of life as the ultimate biosafety tool Markus Schmidt* Organisation for International Dialogue and Conflict Management, Kaiserstr. 50/6, 1070 Vienna, Austria Synthetic biologists try to engineer useful biological search for alternatives. They belong to apparently very systems that do not exist in nature. One of their goals different science fields and their quest for biochemical is to design an orthogonal chromosome different from diversity is driven by different motivations.(1–3) The science DNA and RNA, termed XNA for xeno nucleic acids. XNA exhibits a variety of structural chemical changes relative fields in question include four areas: origin of life, exobiology, to its natural counterparts. These changes make this systems chemistry, and synthetic biology (SB). The ancient novel information-storing biopolymer ‘‘invisible’’ to nat- Greeks, including Aristotle, believed in Generatio spontanea, ural biological systems. The lack of cognition to the the idea that life could suddenly come into being from non- natural world, however, is seen as an opportunity to living matter on an every day basis. Spontaneous generation implement a genetic firewall that impedes exchange of genetic information with the natural world, which means of life, however, was finally discarded by the scientific it could be the ultimate biosafety tool. Here I discuss, why experiments of Pasteur, whose empirical results showed that it is necessary to go ahead designing xenobiological modern organisms do not spontaneously arise in nature from systems like XNA and its XNA binding proteins; what non-living matter. On the sterile earth 4 billion years ago, the biosafety specifications should look like for this however, abiogenesis must have happened at least once, genetic enclave; which steps should be carried out to boot up the first XNA life form; and what it means for the eventually leading to the last universal common ancestor society at large.