Sources of Salinity and Boron in the Gaza Strip: Natural Contaminant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Humanitarian Monitor CAP Occupied Palestinian Territory Number 17 September 2007
The Humanitarian Monitor CAP occupied Palestinian territory Number 17 September 2007 Overview- Key Issues Table of Contents Update on Continued Closure of Gaza Key Issues 1 - 2 Crossings Regional Focus 3 Access and Crossings Rafah and Karni crossings remain closed after more than threemonths. Protection of Civilians 4 - 5 The movement of goods via Gaza border crossings significantly Child Protection 6-7 declined in September compared to previous months. The average Violence & Private 8-9 of 106 truckloads per day that was recorded between 19 June and Property 13 September has dropped to approximately 50 truckloads per day 10 - 11 since mid-September. Sufa crossing (usually opened 5 days a week) Access was closed for 16 days in September, including 8 days for Israeli Socio-economic 12 - 13 holidays, while Kerem Shalom was open only 14 days throughout Conditions the month. The Israeli Civil Liaison Administration reported that the Health 14 - 15 reduction of working hours was due to the Muslim holy month Food Security & 16 - 18 of Ramadan, Jewish holidays and more importantly attacks on the Agriculture crossings by Palestinian militants from inside Gaza. Water & Sanitation 19 Impact of Closure Education 20 As a result of the increased restrictions on Gaza border crossings, The Response 21 - 22 an increasing number of food items – including fruits, fresh meat and fish, frozen meat, frozen vegetables, chicken, powdered milk, dairy Sources & End Notes 23 - 26 products, beverages and cooking oil – are experiencing shortages on the local market. The World Food Programme (WFP) has also reported significant increases in the costs of these items, due to supply, paid for by deductions from overdue Palestinian tax increases in prices on the global market as well as due to restrictions revenues that Israel withholds. -

Protection of Civilians Weekly Report
U N I TOCHA E D Weekly N A Report: T I O 14N MarchS – 20 March 2007 N A T I O N S| 1 U N I E S OFFICE FOR THE COORDINATION OF HUMANITARIAN AFFAIRS P.O. Box 38712, East Jerusalem, Phone: (+972) 2-582 9962 / 582 5853, Fax: (+972) 2-582 5841 [email protected], www.ochaopt.org Protection of Civilians Weekly Report 14 March – 20 March 2007 Of note this week An UNRWA convoy carrying the Director of UNRWA Operations in Gaza was attacked by a group of armed masked gunmen in the northern Gaza Strip. The convoy escaped unharmed despite numerous shots being fired at the vehicle. Gaza Strip − A Palestinian sniper shot and injured a civilian Israeli utility worker in the Nahal Oz area. The military wing of Hamas claimed responsibility. Nine homemade rockets, one of which detonated inside the Gaza Strip, and two mortar shells were fired by Palestinians throughout the week towards Israel. − Four Israeli military boats opened fire and rounded up 14 Palestinian fishing boats in Rafah and forced them to sail towards deeper waters. IDF vessels tied the boats and ordered the fishermen to jump in the water and swim individually towards the military ships. A total of 54 Palestinian fishermen were interrogated before later being released while two others were arrested. − Seven Palestinians were killed this week as a result of internal violence including an eight year-old girl caught in crossfire during a family dispute. − Eight days have passed since the BBC's reporter was abducted in Gaza City. -

Architectural and Functional/Liturgical Development of the North-West Church in Hippos (Sussita) 148 JOLANTA MŁYNARCZYK
CENTRE D’ARCHÉOLOGIE MÉDITERRANÉENNE DE L’ACADÉMIE POLONAISE DES SCIENCES ÉTUDES et TRAVAUX XXII 2008 JOLANTA MŁYNARCZYK Architectural and Functional/Liturgical Development of the North-West Church in Hippos (Sussita) 148 JOLANTA MŁYNARCZYK In July 2007, the eighth season of excavations was completed at the so-called North- West Church at Hippos (Sussita), one of the cities of the Decapolis. The church was explored by a Polish team within the framework of an international project devoted to the unearth- ing of the remains of that Graeco-Roman and Byzantine-Umayyad period town, headed by Arthur Segal of the University of Haifa. Despite the fact that as many as four churches have so far been uncovered at Sussita,1 it is only the North-West Church (NWC) that became one of the examples discussed by A. Ovadiah in his paper listing Byzantine-peri- od churches excavated within the borders of the present-day Israel, in which architectural changes apparently refl ect some liturgical modifi cations.2 Unfortunately, A. Ovadiah’s interpretation of the NWC (published in 2005) not only was based on the reports of the early seasons of our fi eldwork (2002, 2003), but also proved to be rather superfi cial one, a fact which calls for a careful re-examination of the excavation data. Perhaps the most important fact about the NWC is that this has been one of rare in- stances attested for the region of a church that was still active as such during the Um- ayyad period. Archaeological contexts sealed by the earthquake of A.D. -
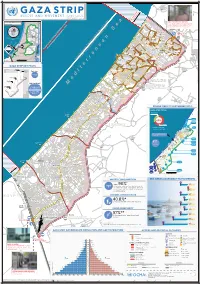
Gaza CRISIS)P H C S Ti P P I U
United Nations Office for the Coordination of Humanitarian Affairs occupied Palestinian territory Zikim e Karmiya s n e o il Z P m A g l in a AGCCESSA ANDZ AMOV EMENTSTRI (GAZA CRISIS)P h c s ti P P i u F a ¥ SEPTEMBER 2014 o nA P N .5 F 1 Yad Mordekhai EREZ CROSSING (BEIT HANOUN) occupied Palestinian territory: ID a As-Siafa OPEN, six days (daytime) a B?week4 for B?3the4 movement d Governorates e e of international workers and limited number of y h s a b R authorized Palestinians including aid workers, medical, P r 2 e A humanitarian cases, businessmen and aid workers. Jenin d 1 e 0 Netiv ha-Asara P c 2 P Tubas r Tulkarm r fo e S P Al Attarta Temporary Wastewater P n b Treatment Lagoons Qalqiliya Nablus Erez Crossing E Ghaboon m Hai Al Amal r Fado's 4 e B? (Beit Hanoun) Salfit t e P P v i Al Qaraya al Badawiya i v P! W e s t R n m (Umm An-Naser) n i o » B a n k a North Gaza º Al Jam'ia ¹¹ M E D I TER RAN EAN Hatabiyya Ramallah da Jericho d L N n r n r KJ S E A ee o Beit Lahia D P o o J g Wastewater Ed t Al Salateen Beit Lahiya h 5 Al Kur'a J a 9 P l D n Treatment Plant D D D D 9 ) D s As Sultan D 1 2 El Khamsa D " Sa D e J D D l i D 0 D s i D D 0 D D d D D m 2 9 Abedl Hamaid D D r D D l D D o s D D a t D D c Jerusalem D D c n P a D D c h D D i t D D s e P! D D A u P 0 D D D e D D D a l m d D D o i t D D l i " D D n . -

How Time Flies When You're Israeli on the One Hand the Region Has Experienced a Sort of Baby Boom
How Time Flies When You're Israeli On the one hand the region has experienced a sort of Baby Boom. On the other hand the number of divorces has increased at an irregular rate, especially in communities near the border. One year since Operation Protective Edge and in the Gaza Envelope settlements they're trying to recover—not a simple matter when there's unanimous agreement that the next round is just around the corner. onday. It's quiet, pretty and clean in the Gaza Envelope. The air is warm and M crisp. The fields bask in the sun, indifferent to what's happening around them. And that's totally fine, because nothing is happening. It's almost one year since Operation Protective Edge. How time flies when you're Israeli. Moti Madmoni of the Schmerling Meat Bar, located at Alonit Junction at the entrance to Gaza, begins to organize his day. During the war, soldiers, journalists and foreigners swarmed here as the skewers of meat flowed out continually. "We did pretty well during the war," he says with a smile. He then describes how hard it was to stand over the grill while his son, a Golani soldier, was fighting on the inside. "But I prefer the quiet, although I don't believe in it. Another round is just a matter of time. This isn't genuine peace—the next battle will come and we'll accept whatever comes with love. We're not leaving. We're here and that's it." I talk with everyone I see, the vast majority of whom don't want to be photographed or quoted by name. -

News of the Israeli-Palestinian Confrontation April 22-29, 2008
Intelligence and Terrorism Information Center Israel Intelligence Heritage and Commemoration Center News of the Israeli-Palestinian Confrontation April 22-29, 2008 Direct hit on a house in Sderot, April 29 The scene of the attack at the Nitzanei Shalom (Photo Zeev Tractman, courtesy of Din industrial area, near Tulkarm (Photo courtesy Veheshbon Communications, Sderot, of ZAKA, April 25). April 29). Overview The main terrorist event this past week was the shooting attack at the Nitzanei Shalom industrial area, near Tulkarm, in which two Israeli civilians were killed. It was another example of terrorist attacks against sites where joint Israeli-Palestinian economic activities are carried out, a clear attempt to damage the interests of the Palestinian people. The high level of rocket fire from the Gaza Strip continued and the IDF continued its counterterrorist activities. Hamas announced that an agreement had been reached in principle with Egypt regarding a gradual lull in the fighting (“Gaza first”) which would later be extended to the West Bank. Hamas regards taking such a step as a means of having the crossings 2 opened on its own terms, and has threatened an escalation in the violence such a lull is not achieved. In the meantime Hamas continues its media campaign to represent the situation in the Gaza Strip as on the brink of collapse, while permitting and even initiating a worsening fuel crisis to back up their campaign. Important Events High level of rocket fire continues Rocket fire into Israel continued during the past week at a relatively high, with 34 identified rocket hits. In addition, 42 mortar shells were fired. -

Autumn 2014 No
Autumn 2014 No. 335 Israel Under Attack WIZO Stands at Israel’s Side Road Safety for Toddlers WIZO Leads the Way There and Back A Story of True Zionism From the Editor Dear Chaverot, to her and the Mexican Jewish community, where there is virtually no assimilation; she also outlines her dreams for Many of the articles in this the future of WIZO Mexico. (page 14) magazine are about people, special people, WIZO people. Miki Doron is a well-known WIZO figure both in Israel and abroad, where he often conducts workshops at conferences. This summer, during Operation How many know that he is a graduate of WIZO Hadassim, Protective Edge, WIZO opened having arrived in Israel alone, as a 15-year-old from Iran. its doors to give shelter and Read Miki’s inspirational story on page 16. peace of mind to hundreds of families from the south of the It’s never too young to learn about road safety. WIZO’s Early country, while our staff and Age Division has a special programme for the toddlers in volunteers all mobilized in our day care centres. (page 18.) different ways. Read all about it on pages 6-11. Many families in Israel cannot afford to give their children Many people talk in despair of the ‘younger’ generation a bar/bat mitzvah celebration. Some of our WIZO branches – many of whom assimilate and have no interest in and in Israel try to provide parties and religious ceremonies for certainly don’t support Israel. But, in this issue we have two needy families in their communities. -

Israel's Rights As a Nation-State in International Diplomacy
Jerusalem Center for Public Affairs Institute for Research and Policy המרכז הירושלמי לענייני ציבור ומדינה )ע"ר( ISRAEl’s RiGHTS as a Nation-State in International Diplomacy Israel’s Rights as a Nation-State in International Diplomacy © 2011 Jerusalem Center for Public Affairs – World Jewish Congress Jerusalem Center for Public Affairs 13 Tel Hai Street, Jerusalem, Israel Tel. 972-2-561-9281 Fax. 972-2-561-9112 Email: [email protected] www.jcpa.org World Jewish Congress 9A Diskin Street, 5th Floor Kiryat Wolfson, Jerusalem 96440 Phone : +972 2 633 3000 Fax: +972 2 659 8100 Email: [email protected] www.worldjewishcongress.com Academic Editor: Ambassador Alan Baker Production Director: Ahuva Volk Graphic Design: Studio Rami & Jaki • www.ramijaki.co.il Cover Photos: Results from the United Nations vote, with signatures, November 29, 1947 (Israel State Archive) UN General Assembly Proclaims Establishment of the State of Israel, November 29, 1947 (Israel National Photo Collection) ISBN: 978-965-218-100-8 TABLE OF CONTENTS Introduction and Overview Ambassador Alan Baker .......................................................................................................................................................................... 5 The National Rights of Jews Professor Ruth Gavison ........................................................................................................................................................................... 9 “An Overwhelmingly Jewish State” - From the Balfour Declaration to the Palestine Mandate -

Table of Contents
Table of Contents Page V. The Threat to Israel’s Civilian Population and Israel’s Civil Defense Measures ............106 A. Life under the Threat of Terrorist Rocket Fire and Cross-Border Tunnel Attacks .................................................................................................................106 B. Israel’s Civil Defence Measures against Rocket and Mortar Attacks .................107 1. Passive Defence Measures .......................................................................107 2. Active Defence Measures (the Iron Dome System) ................................111 C. Harm Caused to Israel’s Civilian Population by Rocket and Mortar Attacks .................................................................................................................112 1. Civilian Deaths and Injuries.....................................................................112 2. Effects on Children, Teenagers and College Students .............................118 3. Effect on the Elderly and People with Disabilities ..................................121 4. Internal Displacement ..............................................................................122 5. Psychological Damage .............................................................................125 6. Economic Damage ...................................................................................132 D. Conclusion ...........................................................................................................136 i V. The Threat to Israel’s Civilian Population -
ILH MAP 2014 Site Copy
Syria 99 a Mt.Hermon M 98 rail Odem Lebanon T O Rosh GOLAN HEIGHTS 98 Ha-Nikra IsraelNational 90 91 C Ha-Khula 899 Tel Hazor Akhziv Ma’alot Tarshiha 1 Nahariya 89 89 Katzrin More than a bed to sleep in! L. 4 3 888 12 Vered Hagalil 87 Clil Yehudiya Forest Acre E 85 5 4 Almagor 85 85 6 98 Inbar 90 Gamla 70 Karmiel Capernaum A 807 79 GALILEE 65 -212 meters 92 Givat Yoav R 13 -695 11 2 70 79 Zippori 8 7 75 Hilf Tabash 77 2 77 90 75 Nazareth 767 Khamat Israel’s Top 10 Nature Reserves & National Parks 70 9 Yardenit Gader -IS Mt. Carmel 10 Baptismal Site 4 Yoqneam Irbid Hermon National Park (Banias) - A basalt canyon hiking trail leading Nahal 60 S Me’arot to the largest waterfall in Israel. 70 Afula Zichron Ya’acov Megiddo 65 90 Yehudiya Forest Nature Reserve - Come hike these magnicent 71 trails that run along rivers, natural pools, and waterfalls. 60 Beit Alfa Jisr Az-Zarqa 14 6 Beit 65 Gan Shean Zippori National Park - A site oering impressive ruins and Caesarea Um El-Fahm Hashlosha Beit mosaics, including the stunning “Mona Lisa of the Galilee”. 2 Shean Jordan TEL Hadera 65 River Jenin Crossing Caesarea National Park - Explore the 3500-seat theatre and 6 585 S other remains from the Roman Empire at this enchanting port city. Jarash 4 Jerusalem Walls National Park - Tour this amazing park and view Biblical 60 90 Netanya Jerusalem from the city walls or go deep into the underground tunnels. -
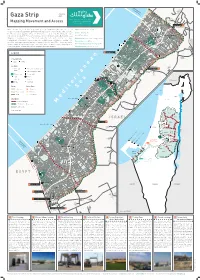
Gaza Strip 2020 As-Siafa Mapping Movement and Access Netiv Ha'asara Temporary
Zikim Karmiya No Fishing Zone 1.5 nautical miles Yad Mordekhai January Gaza Strip 2020 As-Siafa Mapping Movement and Access Netiv Ha'asara Temporary Ar-Rasheed Wastewater Treatment Lagoons Sources: OCHA, Palestinian Central Bureau of Statistics of Statistics Bureau Central OCHA, Palestinian Sources: Erez Crossing 1 Al-Qarya Beit Hanoun Al-Badawiya (Umm An-Naser) Erez What is known today as the Gaza Strip, originally a region in Mandatory Palestine, was created Width 5.7-12.5 km / 3.5 – 7.7 mi through the armistice agreements between Israel and Egypt in 1949. From that time until 1967, North Gaza Length ~40 km / 24.8 mi Al- Karama As-Sekka the Strip was under Egyptian control, cut off from Israel as well as the West Bank, which was Izbat Beit Hanoun al-Jaker Road Area 365 km2 / 141 m2 Beit Hanoun under Jordanian rule. In 1967, the connection was renewed when both the West Bank and the Gaza Madinat Beit Lahia Al-'Awda Strip were occupied by Israel. The 1993 Oslo Accords define Gaza and the West Bank as a single Sheikh Zayed Beit Hanoun Population 1,943,398 • 48% Under age 17 July 2019 Industrial Zone Ash-Shati Housing Project Jabalia Sderot territorial unit within which freedom of movement would be permitted. However, starting in the camp al-Wazeer Unemployment rate 47% 2019 Q2 Jabalia Camp Khalil early 90s, Israel began a gradual process of closing off the Strip; since 2007, it has enforced a full Ash-Sheikh closure, forbidding exit and entry except in rare cases. Israel continues to control many aspects of Percentage of population receiving aid 80% An-Naser Radwan Salah Ad-Deen 2 life in Gaza, most of its land crossings, its territorial waters and airspace. -

Israeli Settler-Colonialism and Apartheid Over Palestine
Metula Majdal Shams Abil al-Qamh ! Neve Ativ Misgav Am Yuval Nimrod ! Al-Sanbariyya Kfar Gil'adi ZZ Ma'ayan Baruch ! MM Ein Qiniyye ! Dan Sanir Israeli Settler-Colonialism and Apartheid over Palestine Al-Sanbariyya DD Al-Manshiyya ! Dafna ! Mas'ada ! Al-Khisas Khan Al-Duwayr ¥ Huneen Al-Zuq Al-tahtani ! ! ! HaGoshrim Al Mansoura Margaliot Kiryat !Shmona al-Madahel G GLazGzaGza!G G G ! Al Khalsa Buq'ata Ethnic Cleansing and Population Transfer (1948 – present) G GBeGit GHil!GlelG Gal-'A!bisiyya Menara G G G G G G G Odem Qaytiyya Kfar Szold In order to establish exclusive Jewish-Israeli control, Israel has carried out a policy of population transfer. By fostering Jewish G G G!G SG dGe NG ehemia G AGl-NGa'iGmaG G G immigration and settlements, and forcibly displacing indigenous Palestinians, Israel has changed the demographic composition of the ¥ G G G G G G G !Al-Dawwara El-Rom G G G G G GAmG ir country. Today, 70% of Palestinians are refugees and internally displaced persons and approximately one half of the people are in exile G G GKfGar GB!lGumG G G G G G G SGalihiya abroad. None of them are allowed to return. L e b a n o n Shamir U N D ii s e n g a g e m e n tt O b s e rr v a tt ii o n F o rr c e s Al Buwayziyya! NeoG t MG oGrdGecGhaGi G ! G G G!G G G G Al-Hamra G GAl-GZawG iyGa G G ! Khiyam Al Walid Forcible transfer of Palestinians continues until today, mainly in the Southern District (Beersheba Region), the historical, coastal G G G G GAl-GMuGftskhara ! G G G G G G G Lehavot HaBashan Palestinian towns ("mixed towns") and in the occupied West Bank, in particular in the Israeli-prolaimed “greater Jerusalem”, the Jordan G G G G G G G Merom Golan Yiftah G G G G G G G Valley and the southern Hebron District.