Immunohistochemical Analysis of S100-Positive Epidermal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Storiform Collagenoma: Case Report Colagenoma Estoriforme: Relato De Caso
CASE REPORT Storiform collagenoma: case report Colagenoma estoriforme: relato de caso Guilherme Flosi Stocchero1 ABSTRACT INTRODUCTION Storiform collagenoma is a rare tumor, which originates from the Storiform collagenoma or sclerotic fibroma is a rare proliferation of fibroblasts that show increased production of type-I benign skin tumor that usually affects young adults collagen. It is usually found in the face, neck and extremities, but and middle-age individuals of both sexes. This tumor is it can also appear in the trunk, scalp and, less frequently, in the slightly predominant in women. Storiform collagenoma oral mucosa and the nail bed. It affects both sexes, with a slight female predominance. It may be solitary or multiple, the latter being appears as a small papule or solid fibrous nodule. an important marker for Cowden syndrome. It presents as a painless, It is well-circumscribed, pink, whitish or skin color, solid nodular tumor that is slow-growing. It must be considered in the painless and of slow-growing. This tumor is often differential diagnosis of other well-circumscribed skin lesions, such as found in face and limbs, but it can also appears in dermatofibroma, pleomorphic fibroma, sclerotic lipoma, fibrolipoma, the chest, scalp and, rarely, in oral mucosa and nail giant cell collagenoma, benign fibrous histiocytoma, intradermal Spitz bed. Storiform collagenoma often appears as single nevus and giant cell angiohistiocytoma. tumor, and the occurrence of multiple tumors is an important indication of Cowden syndrome, which is Keywords: Collagen; Hamartoma; Skin neoplasms; Fibroma; Skin; Case a heritage genodermatosis of autosomal dominant reports condition.(1-4) Storiform collagenoma has as differential diagnosis other well-circumscribed skin tumors such RESUMO as dermatofibroma, pleomorphic fibroma, sclerotic O colagenoma estoriforme é um tumor raro originado a partir da lipoma, fibrolipoma, giant cell collagenoma, benign proliferação de fibroblastos com produção aumentada de colágeno tipo I. -

Dermatofibrosarcoma Protuberans and Dermatofibroma: Dermal Dendrocytomas? a Ultrastructural Study
Dermatofibrosarcoma Protuberans and Dermatofibroma: Dermal Dendrocytomas? A Ultrastructural Study Hugo Dominguez-Malagon, M.D., Ana Maria Cano-Valdez, M.D. Department of Pathology, Instituto Nacional de Cancerología, Mexico. ABSTRACT population of plump spindled cells devoid of cell processes, these cells contained intracytoplasmic lipid droplets and rare Dermatofibroma (DF) and Dermatofibrosarcoma subplasmalemmal densities but lacked MVB. Protuberans (DFSP) are dermal tumors whose histogenesis has not been well defined to date. The differential diagnosis in most With the ultrastructural characteristics and the constant cases is established in routine H/E sections and may be confirmed expression of CD34 in DFSP, a probable origin in dermal by immunohistochemistry; however there are atypical variants dendrocytes is postulated. The histogenesis of DF remains of DF with less clear histological differences and non-conclusive obscure. immunohistochemical results. In such cases electron microscopy studies may be useful to establish the diagnosis. INTRODUCTION In the present paper the ultrastructural characteristics of 38 The histogenesis or differentiation of cases of DFSP and 10 cases of DF are described in detail, the Dermatofibrosarcoma Protuberans (DFSP) and objective was to identify some features potentially useful for Dermatofibroma (DF) is controversial in the literature. For differential diagnosis, and to identify the possible histogenesis of DFSP diverse origins such as fibroblastic,1 fibro-histiocytic2 both neoplasms. Schwannian,3 myofibroblastic,4 perineurial,5,6 and endoneurial (7) have been postulated. DFSP in all cases was formed by stellate or spindled cells with long, slender, ramified cell processes joined by primitive junctions, Regarding DF, most authors are in agreement of a subplasmalemmal densities were frequently seen in the processes. -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

Basal Cell Carcinoma Associated with Non-Neoplastic Cutaneous Conditions: a Comprehensive Review
Volume 27 Number 2| February 2021 Dermatology Online Journal || Review 27(2):1 Basal cell carcinoma associated with non-neoplastic cutaneous conditions: a comprehensive review Philip R Cohen MD1,2 Affiliations: 1San Diego Family Dermatology, National City, California, USA, 2Touro University California College of Osteopathic Medicine, Vallejo, California, USA Corresponding Author: Philip R Cohen, 10991 Twinleaf Court, San Diego, CA 92131-3643, Email: [email protected] pathogenesis of BCC is associated with the Abstract hedgehog signaling pathway and mutations in the Basal cell carcinoma (BCC) can be a component of a patched homologue 1 (PCTH-1) transmembrane collision tumor in which the skin cancer is present at tumor-suppressing protein [2-4]. Several potential the same cutaneous site as either a benign tumor or risk factors influence the development of BCC a malignant neoplasm. However, BCC can also concurrently occur at the same skin location as a non- including exposure to ultraviolet radiation, genetic neoplastic cutaneous condition. These include predisposition, genodermatoses, immunosuppression, autoimmune diseases (vitiligo), cutaneous disorders and trauma [5]. (Darier disease), dermal conditions (granuloma Basal cell carcinoma usually presents as an isolated faciale), dermal depositions (amyloid, calcinosis cutis, cutaneous focal mucinosis, osteoma cutis, and tumor on sun-exposed skin [6-9]. However, they can tattoo), dermatitis, miscellaneous conditions occur as collision tumors—referred to as BCC- (rhinophyma, sarcoidal reaction, and varicose veins), associated multiple skin neoplasms at one site scars, surgical sites, systemic diseases (sarcoidosis), (MUSK IN A NEST)—in which either a benign and/or systemic infections (leischmaniasis, leprosy and malignant neoplasm is associated with the BCC at lupus vulgaris), and ulcers. -

Robust Surgical Approach for Cutaneous Neurofibroma in Neurofibromatosis Type 1
Robust surgical approach for cutaneous neurofibroma in neurofibromatosis type 1 Bahir H. Chamseddin, … , Juan Vega, Lu Q. Le JCI Insight. 2019. https://doi.org/10.1172/jci.insight.128881. Clinical Medicine In-Press Preview Dermatology Neuroscience BACKGROUND. Cutaneous neurofibromas (cNF) are physically disfiguring, painful, and cause extensive psychologic harm in patients with neurofibromatosis type 1 (NF1). There is currently no effective medical treatment and surgical procedures are inaccessible to most NF1 patients globally. OBJECTIVE. While research is underway to find an effective medical treatment for cNF, there is an urgent need to develop surgical approach that is accessible to all NF1 patients in the world with the skill set and equipment found in most general medical office settings. Here, we present a robust surgical approach to remove cNF that does not require sterile surgical field, utilizes accessible clinical equipment, and can be performed by any health care providers including family practitioners, and physician assistants. METHODS. In a prospective case-series, patients with NF1 underwent this surgical procedure which removes multiple cutaneous neurofibromas. The Dermatology Life Quality Index was given to subjects before and after the procedure as surrogate for patient satisfaction. RESULTS. 83 tumors were removed throughout the body from twelve individuals. Examination at follow-up visits revealed well-healed scars without infection or adverse events including aberrant scarring. Patient satisfaction with the procedure was high with significant improvements […] Find the latest version: https://jci.me/128881/pdf Robust Surgical Approach for Cutaneous Neurofibroma in Neurofibromatosis Type 1 Bahir H. Chamseddin1, La’Nette Hernandez1, Dezehree Solorzano1, Juan Vega1, Lu Q. -

Seborrheic Keratosis
Benign Epidermal and Dermal Tumors REAGAN ANDERSON, DO- PROGRAM DIRECTOR, COLORADO DERMATOLOGY INSTITUTE, RVU PGY3 RESIDENTS- JONATHAN BIELFIELD, GEORGE BRANT PGY2 RESIDENT- MICHELLE ELWAY Seborrheic Keratosis Common benign growth seen after third/fourth decade of life Ubiquitous among older individuals Tan to black, macular, papular, or verrucous lesion Occur everywhere except palms, soles, and mucous membranes Can simulate melanocytic neoplasms Pathogenesis: Sun exposure- Australian study found higher incidence in the head/neck Alteration in distribution of epidermal growth factors Somatic activating mutations in fibroblast growth factor receptor and phosphoinositide-3-kinase Seborrheic Keratosis Sign of Leser-Trelat: Rare cutaneous marker of internal malignancy • Gastric/colonic adenocarcinoma, breast carcinoma, and lymphoma m/c • Abrupt increase in number/size of SKs that can occur before, during, or after an internal malignancy is detected • 40% pruritus • M/C location is the back • Malignant acanthosis nigricans may also appear in 20% of patients • Should resolve when primary tumor is treated, and reappear with recurrence/mets Seborrheic Keratosis 6 Histologic types Acanthotic Hyperkeratotic Reticulated Irritated Clonal Melanoacanthoma Borst-Jadassohn phenomenon Well-demarcated nests of keratinocytes within the epidermis Seborrheic Keratoses Treatment Reassurance Irritated SKs (itching, catching on clothes, inflamed) Cryotherapy, curettage, shave excision Pulsed CO2, erbium:YAG lasers Electrodessication Flegel -

Genital Lesions
Nick Van Wagoner, MD PhD Department of Infectious Diseases University of Alabama at Birmingham By the end of the lecture, the participant should be familiar with: Commonly used dermatological terms Common dermatological conditions seen in STD Clinics Dematology language Genital anatomy Categories of lesions Dermatological conditions seen in the STD clinic Not all individuals presenting to STD Clinics have STDs. Consider other causes for symptoms. History and examination are important in diagnosing non-STD genital findings. How long has it been present? What have you been doing to the skin in that area? Do you have any skin trouble anywhere else? Does this this look like an STD? Has this person been treated for STDs? Did it help? Know your limitations: Ask and Refer Symptoms perceived to be an STD Drips Discharge Dysuria Pain Redness Ulcers Bumps “I have spots on my head where hair won’t grow.” “I have a bump on my arm that hurts.” “I have painful sores on my back. I was told it was herpes.” History Exam Treat STD Reassure Not an STD Treat Refer Macule Patch area of color change area of color change <1.5cm, nonpalpable >1.5cm, nonpalpable From: www.acponline.org From: www.merck.com From: www.dermatology.cdlib.org From: www/missinglink.ucsf.edu From: www.visualdxhealth.com From: www.dermubc.ca From: www.dermubc.ca Papule Plaque area of elevation area of elevation <1.0cm, palpable >1.0cm, palpable elevated From: www.dermatology,about.com From: www.mycology.adelaide.edu/au/gallery From: www/missinglink.ucsf.edu www.dermatology.about.com -

Oral Benign Fibrous Histiocytoma – a Review of Literature from 1964-2016
Review Article Adv Dent & Oral Health Volume 8 Issue 5 - May 2018 Copyright © All rights are reserved by Prasanna Kumar DOI: 10.19080/ADOH.2018.08.555748 Oral Benign Fibrous Histiocytoma – A Review of Literature from 1964-2016 Prasanna Kumar* Rajiv Gandhi University of Health Sciences, India Submission: March 18, 2017; Published: May 17, 2018 *Corresponding author: Prasanna Kumar, Rajiv Gandhi University of Health Sciences, Dept of Oral & MAxillofacial Surgery, Sullia, Bangalore, India, Tel: ; Email: Introduction It all began way before the 1960’s, however on one fateful incidence of metastasis or recurrences if any. day in the year of 1961, Kauffman ST and Stout AP changed the the diagnostic techniques, current protocols in treatment and Discussion way the world looked at fibrous soft tissue tumours by being the separate clinical entity [1]. first to report about Fibrous Histiocytoma and recognising it as a the mean age of patients was 55 years ranging from 12 to 71 years. The oral and perioral cases of BFH, Gray et al. [8] found that Benign fibrous histiocytoma designates a group of quasi- [9] Women are more frequently affected than men. Bielamowicz et differentiation. Whether the lesions originate from histiocytic or neoplastic lesions that show both fibroblastic and histiocytic al. [9] in their study of BFH in the head and neck region found M: F ratio of 2.5:1 [10]. fibroblasticSome experts tissues hypothesize has not been that clearly the determined cells originate yet [2].from the depending upon the location, duration and possible aetiology. The clinical picture as seen in literature varies significantly while others argue that immunohistochemical evidence of factor tissue histiocytes and then assume fibroblastic properties [3] Various causes have been speculated in the aetiology of BFH XII a positivity favours a dermal dendrocytic cell origin [4]. -
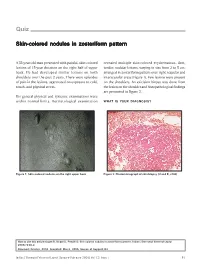
Skin-Colored Nodules in Zosteriform Pattern
Quiz Skin-colored nodules in zosteriform pattern A 33-year-old man presented with painful, skin-colored revealed multiple skin-colored erythematous, firm, lesions of 15-year duration on the right half of upper tender, nodular lesions, varying in size from 2 to 5 cm, back. He had developed similar lesions on both arranged in zosteriform pattern over right scapular and shoulders over the past 2 years. There were episodes interscapular areas (Figure 1). Few lesions were present of pain in the lesions, aggravated on exposure to cold, on the shoulders. An exicision biopsy was done from touch, and physical stress. the lesion on the shoulder and histopathological findings are presented in Figure 2. His general physical and systemic examinations were within normal limits. Dermatological examination WHAT IS YOUR DIAGNOSIS? Figure 1: Skin-colored nodules on the right upper back Figure 2: Photomicrograph of skin biopsy (H and E, x100) How to cite this article: Gupta R, Singal A, Pandhi D. Skin colored nodules in zosteriforms pattern. Indian J Dermatol Venereol Leprol 2005;72:81-2. Received: October, 2004. Accepted: March, 2005. Source of Support: Nil. Indian J Dermatol Venereol Leprol|January-February 2006|Vol 72|Issue 1 81 Quiz Diagnosis: Type-2 segmental leiomyoma cutis via sympathetic nervous system. Muscle contraction ensues, triggered by the influx of calcium ions. Hence, Histopathological findings nifedipine, a calcium channel blocker has a role in Biopsy showed a tumor composed of irregularly relieving pain associated with cutaneous leiomyoma. arranged smooth-muscle fibers, with elongated nuclei and rounded ends, interlaced with variable amounts of Several conditions are associated with piloleiomyoma,[4] collagen in the reticular dermis. -

Dermoscopy As an Adjuvant Tool for Detecting Skin Leiomyomas In
Diluvio et al. BMC Dermatology 2014, 14:7 http://www.biomedcentral.com/1471-5945/14/7 CASE REPORT Open Access Dermoscopy as an adjuvant tool for detecting skin leiomyomas in patient with uterine fibroids and cerebral cavernomas Laura Diluvio1*, Claudia Torti1, Alessandro Terrinoni2, Eleonora Candi2, Raffaella Piancatelli3, Emilio Piccione3, Evelin Jasmine Paternò4, Sergio Chimenti1, Augusto Orlandi5, Elena Campione1† and Luca Bianchi1† Abstract Background: Hereditary syndromes frequently need the cooperation of different specialties to increase diagnostic competence. Multiple cutaneous and uterine leiomyomatosis syndrome is a rare autosomal dominant disorder caused by the mutations of the fumarate hydratase gene, demonstrated in 80 to 100 percent of affected individuals. This can be linked to an increased risk of renal cancer in both sexes. The skin involvement is described to highlight the diagnostic role of the cutaneous counterpart in identifying this rare syndrome. Case presentation: A 37-year-old woman suffering from several uterine fibroids presented multiple, painful, papulo-nodules on her left subscapular side, both forearms and legs. The patient underwent surgery on six lesions: five were leiomyomas, whilst one was a dermatofibroma. Genetic sequencing did not evidence known fumarate hydratase gene mutations. Dermoscopy showed a brown delicate pigmented network and included leiomyomas among the non-melanocytic benign skin tumours featuring a dermatofibroma-like pattern. Abdominal computerized-tomography scan did not reveal renal cancer, but brain magnetic resonance imaging showed one asymptomatic cerebral cavernoma. The patient benefited from the surgical removal of the five larger cutaneous lesions and from gabapentin, which relieved her pain. Conclusions: This observation highlights the usefulness of dermoscopy in the diagnosis of cutaneous leiomyomas disclosing multiple cutaneous and uterine leiomyomatosis syndrome. -

Dermoscopic Patterns in Active and Regressive Lichen Planus
Journal of Pakistan Association of Dermatologists. 2020; 30(3): 461-467. Original Article Dermoscopic patterns in active and regressive lichen planus Aisha Ghias, Atiya Arshad, Madiha Sanai, Aneela Asghar, Saelah Batool*, Zunaira Arshad, Tahir Jameel Ahmad Department of Dermatology, KEMU/ Mayo Hospital, Lahore. * Department of Dermatology, SIMS/ Services Hospital, Lahore. Abstract Objective To determine various dermoscopic patterns in active and regressive Lichen Planus. Methods It was an observational study carried out at department of dermatology unit-II, Mayo Hospital Lahore. A total of 70 dermoscopic images from 35 patients (20 females, 15 males) were studied. Their clinical pictures were taken with iPhone 6 and patients diagnosed both clinically and histologically of lichen planus were enrolled in the study. Dermoscopic pictures were taken at the same time with fireflypro DE 350 model, a polarized dermoscopic device, using both optical and digital magnification. Clinical and dermoscopic data of both active and regressive Lichen Planus was compiled separately. Predominant patterns were described keeping in mind the internationally accepted terminology & criteria. Results 40 out of 70 images belonged to patients with clinically active LP, while 30 images showed features of regressive LP. Predominant features in active LP included wikhams stria (WS), vascular structures and various forms of pigmentation. WS and vascular structures are absent in majority of treated or regressive cases. Conclusion Dermoscopy is a reliable non-invasive tool in differentiating active from regressive LP. Key words Dermoscopy, lichen planus, active, regressive. Introduction suspicious lesions has already been established but in “skin of colour” a lot is yet to be seen. Dermoscopy is a relatively new diagnostic tool There are two basic types of dermoscopic used worldwide for the detailed assessment and devices, polarized and non-polarized. -

Common Skin Growths and Tumours
Skin Tags / Fibroepithelial Polyps • Seborrheic keratoses can increase in numbers with Sebaceous Hyperplasia age and with prolonged sun exposure. • Skin tags are common, benign flesh-coloured to • Sebaceous hyperplasia are benign skin growths brown skin growths. • Treatment is usually not necessary unless they made up of enlarged oil (sebaceous) glands. They become itchy, irritated or of cosmetic concern. occur in adults and are more common in males. • There is an increased risk in overweight individuals, Treatment options include cryotherapy (liquid pregnant women and those with other family • They present as small, flesh-coloured to yellow nitrogen), scraping of the skin (curettage or members who have skin tags. bumps, most commonly found on the face. cautery) or laser therapy. • They are commonly found in skin folds such as the • Sometimes, they can mimic skin cancer and a • Sometimes a darkly pigmented seborrheic neck, armpits, and groin, although they can occur small skin biopsy may be needed for microscopic keratosis may look like a cancerous mole. In these Common Skin just about anywhere on the body. examination to confirm the diagnosis. cases, a small skin biopsy may need to be taken Growths and Tumours • Occasionally, they may twist and strangulate their for microscopic examination. The risk of recurrence • Treatment is purely for cosmetic reason, and blood supply, resulting in pain. Trauma and irritation is high. include cryotherapy (liquid nitrogen), electrosurgery can occur from rubbing against clothing or jewelry. or laser ablation. • Skin tags may be left alone. However,they can be Cherry Angioma removed with simple snip/ shave excision, lasers or • Cherry angiomas are small, benign growths made electrocautery.