Macroscopic Anatomy Embryonic Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gross Anatomy of the Suprarenal Glands
Edited by: Malak Shalfawi, Noor Adnan Gross Anatomy of the suprarenal glands 5/10/2020 Dr. shatarat. The University of Jordan In the sagittal section below, you can see the retroperitoneal space (encircled by a blue line), which contains structures that lie deep on the posterior abdominal wall and are called retroperitoneal structures, they are the kidneys and suprarenal (adrenal) glands. ➔ The adrenal glands are two small triangular structures located retroperitoneally at the upper poles of the kidneys. [notice the black arrow] 5/10/2020 Dr. shatarat. The University of Jordan You can again notice the kidneys (lying on the posterior abdominal wall and covered by fat), The peritoneum and retroperitoneal space. ➔ The adrenal glands are covered with a thick connective tissue capsule from which the trabeculae extend into the parenchyma carrying blood vessels and nerves. **Extra note: all soft structures in the abdomen, such as the spleen, kidneys and suprarenal glands, have hilum into which all blood vessels and nerve supply getting in or out of them. But each one of these soft structures has its specific modifications on its In this section, you can see the hilum. For example, the ureter getting vertebral column and the muscles of out from the kidneys. the posterior abdominal wall (quadratus lumborum and Psoas 5/10/2020 Dr. shatarat. The University of Jordan major) ➔ Adrenal glands are found on the posterior parietal wall, on each side of the vertebral column, at the level of the 11th thoracic rib and lateral to the first lumber vertebra. They are in the upper part of the abdomen, almost near the diaphragm, NOT in the middle and NOT inferior!!!! ➔ They have flattened triangular shape and are embedded in the perirenal fat at the superior poles of the kidneys. -

Histogenesis of Suprarenal Glands at Different Gestational Age Groups
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Histogenesis of suprarenal glands at different gestational age groups Ravindra Kumar Boddeti1, Subhadra Devi Velichety2 1Lecturer, 2Professor and Head, Department of Anatomy, Sri Padmavathi Medical College for Women, Sri Venkateswara Institute of Medical Sciences, SVIMS University, Tirupathi, Andhra Pradesh, India Submitted: 22-02-2019 Revised: 10-03-2019 Published: 01-05-2019 ABSTRACT Background: The human foetal suprarenal gland is structurally variant from its adult Access this article online counterpart. The most distinctive features of human foetal suprarenal gland and histologically Website: unique foetal zone, was described first by Elliott and Armour in 1911. After the first trimester, the centrally located foetal zone accounts for most of the foetal adrenal mass. The outer zone http://nepjol.info/index.php/AJMS of the foetal suprarenal gland is called the “definitive zone or neo cortex”; this zone likely DOI: 10.3126/ajms.v10i3.22820 gives rise to the adult adrenal glomerulosa. A third zone called “transitional zone”, lies just E-ISSN: 2091-0576 2467-9100 between the neocortex and foetal zone and is believed to develop into the zona fasciculata. P-ISSN: Aims and Objectives: The current study was designed to study the histogenesis of suprarenal glands at different gestational age groups. Materials and Methods: Twenty-eight formalin preserved dead embryos and foetuses of both sexes, were obtained from the Govt. Maternity Hospital & S.V.Medical College, Tirupati, Andhra Pradesh, India. Specimens were grouped according to their gestational age groups (A,B,C,D) A= 0-12 weeks, B= 13-24 weeks, C= 25-36 weeks and D= more than 36 weeks of gestation. -

Pig Gonads, Adrenal Glands and Brain C
Immunoreactive cytochrome P-45017\g=a\in rat and guinea- pig gonads, adrenal glands and brain C. Le Goascogne1, N. Sanan\l=e'\s1, M. Gou\l=e'\zou1, S. Takemori2, S. Kominami2, E. E. Baulieu1 and P. Robel1 1INSERM U33, Communications Hormonales, and Faculté de Médecine, Université Paris-sud, Lab Hormones F-94275 Bicêtre Cedex France 2 Faculty of Integrated Arts and Sciences, Hiroshima University, Hiroshima 730, Japan Summary. The cytochrome P-45017\g=a\-hydroxylase, 17\ar=r\20lyase (P-45017\g=a\) is the key enzyme responsible for the biosynthesis of androgens in steroidogenic organs. Its cellular localization has been examined with an immunohistochemical technique. In immature rat ovary, P-45017\g=a\was first detected in sparse interstitial cells on postnatal Day 8. The number of immunoreactive interstitial cells increased thereafter and the intensity of P-45017\g=a\staining in these cells was highest at 3 weeks of age. The intensity of staining then started to decline and was very faint at Day 35. From 6 weeks on, the distribution of immunoreactive P-45017\g=a\was of the adult type: it was detected exclusively in the thecal cells of the large antral, preovulatory, follicles. P-45017\g=a\was not detectable during pregnancy except on the day of parturition, when thecal cells were transiently immunoreactive. The staining had vanished 24 h after delivery. Human chorionic gonadotrophin (hCG), injected into immature females on Days 24 to 26, induced P-45017\g=a\prematurely in thecal cells. When injected on Days 12 to 14 of pregnancy, hCG also induced P-45017\g=a\in the thecal cells surrounding the largest follicles, whereas the interstitial and luteal cells were not immunostained. -

PKA Signaling Drives Reticularis Differentiation and Sexually Dimorphic Adrenal Cortex Renewal
PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal Typhanie Dumontet, … , Pierre Val, Antoine Martinez JCI Insight. 2018;3(2):e98394. https://doi.org/10.1172/jci.insight.98394. Research Article Development Endocrinology The adrenal cortex undergoes remodeling during fetal and postnatal life. How zona reticularis emerges in the postnatal gland to support adrenarche, a process whereby higher primates increase prepubertal androgen secretion, is unknown. Using cell-fate mapping and gene deletion studies in mice, we show that activation of PKA has no effect on the fetal cortex, while it accelerates regeneration of the adult cortex, triggers zona fasciculata differentiation that is subsequently converted into a functional reticularis-like zone, and drives hypersecretion syndromes. Remarkably, PKA effects are influenced by sex. Indeed, testicular androgens increase WNT signaling that antagonizes PKA, leading to slower adrenocortical cell turnover and delayed phenotype whereas gonadectomy sensitizes males to hypercorticism and reticularis-like formation. Thus, reticularis results from ultimate centripetal conversion of adult cortex under the combined effects of PKA and cell turnover that dictate organ size. We show that PKA-induced progenitor recruitment is sexually dimorphic and may provide a paradigm for overrepresentation of women in adrenal diseases. Find the latest version: https://jci.me/98394/pdf RESEARCH ARTICLE PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal Typhanie Dumontet,1 Isabelle Sahut-Barnola,1 Amandine Septier,1 Nathanaëlle Montanier,1 Ingrid Plotton,2 Florence Roucher-Boulez,2 Véronique Ducros,3 Anne-Marie Lefrançois-Martinez,1 Jean-Christophe Pointud,1 Mohamad Zubair,4 Ken-Ichirou Morohashi,4 David T. Breault,5,6 Pierre Val,1 and Antoine Martinez1 1GReD, Université Clermont Auvergne, CNRS, INSERM, Clermont-Ferrand, France. -

Adrenal Gland
ADRENAL GLAND Objectives: ◧ Editing file • Differentiate between adrenal cortex ◧ Important and medulla. ◧ Doctor notes / Extra • Identify the histological features of each cortical zone and its cells. • Identify the histological features of the medullary cells. 438 Histology Team Endocrine Block Zona glomerulosa Stroma Cortex Zona fasciculata Parenchyma Zona reticularis Adrenal gland Adrenal Medulla The adrenal cortex layers have 5 features in common: extra but important 1. Suprarenal artery 1- Acidophilic cytoplasm 2. Capsule 2- Abundant SER 3. Zona glomerulosa 3- Numerous mitochondria 4. Zona fasciculata 5. Zona reticularis 4- Mitochondrial cristae is tubular 6. Medulla 5- few Droplet of lipids ( expect Zona fasciculata rich in lipids) 7. Central vein of medulla 438 Histology Team - Endocrine Block 2 Adrenal Cortex Zona fasciculata Zona glomerulosa Zona reticularis (spongiocytes) ● formed of clusters of small • It is the intermediate and the largest •It is the innermost layer of adrenal columnar cells that are rich in layer of the cortex. cortex. SER and mitochondria. • It is formed of columns of large •It is formed of anastomosing ● Produces mineralocorticoids polyhedral cells that are separated cords of deep acidophilic cells. e.g. aldosterone hormone by longitudinal sinusoidal capillaries. •Its cells contains few lipofuscin (Reabsorb all the remaining • Its cells are rich in lipids, so they and lipid droplets. sodium, and passively the appear empty in sections chloride, from the lumen of the (spongiocytes). •The cells secrete androgens. distal renal tubules into the renal • Its cells are rich in mitochondria (with interstitium. In addition, tubular cristae),SER and lipofuscin potassium and hydrogen ions pigments. are actively secreted into the lumen). -
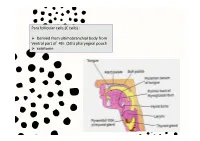
Para Follicular Cells (C Cells)
Para follicular cells (C cells) : Derived from ultimobranchial body from Ventral part of 4th (5th) pharyngeal pouch calcitonin Thyroglossal cyct: path of thyroid descending Position of occur: Inf. To the body of hyoid 50% Base of tongue Close to thyroid cartilage Thyroglossal fistula Abberant thyroid tissue: path of thyroid descending Base of tongue Histology of the thyroid gland Thyroid gland: parafollicular cells: Capsule / trabeculae Follicles / reticular fiber / Decreased Ca+ in blood by 2 ways: basal lamina Follicular cells/ basal lamina 1. Transport Ca from blood to musculoskeletal system parafollicular cells 2. Prevent bone absorption by osteoclast cells Colloid (hormone storage) Produce energy & temperature for body activity Histology of the thyroid gland Thyroid hormones: Tri-iodothyronin Tetra-iodothyronin Calcitonin Fenestrated capillary Synthesis and secretion of thyroid hormones T3 and T4 Synthesis and secretion of thyroid hormones T3 and T4 Synthesis and mechanism of action of calcitonin Gravies disease (exophthalmic goiter / toxic goiter): excessive amounts of thyroid hormones are released into the circulation detectable levels of autoantibodies abnormal immunoglobulins (IgG) bind to the TSH receptor in-creased thyroid hormone secretion Because of negative feedback, the levels of TSH in the circulation are usually normal Hypertrophy thyroid hormone Is abnormally high range increased metabolism Features: weight loss / excessive sweating / tachycardia /nervousness / protrusion of the eyeballs / retraction of the -

Anatomy of Endocrine System
Anatomy of Endocrine system Introduction, Pituitary gland and Thyroid gland Prepared by Dr. Payal Jain Endocrine System I. Introduction A. Considered to be part of animals communication system 1. Nervous system uses physical structures for communication 2. Endocrine system uses body fluids to transport messages (hormones) II. Hormones A. Classically, hormones are defined as chemical substances produced by ductless glands and secreted into the blood supply to affect a tissue distant from the gland, but now it is understood that hormones can be produced by single cells as well. 1. epicrine a. hormones pass through gap junctions of adjacent cells without entering extracellular fluid 2. paracrine a. hormones diffuse through interstitial fluid (e.g. prostaglandins) 3. endocrine a. hormones are delivered via the bloodstream (e.g. growth hormone Different endocrine glands with cell Organ Division arrangement Cell arrangement/morphology Hormone Hypophysis Adenohypophysis Pars distalis Cells in cords around large-bore capillaries: Acidophils Growth hormone, prolactin Basophils ACTH, TSH, FSH, LH Pars intermedia Mostly basophilic cells around ACTH, POMC cystic cavities Pars tuberalis Narrow sleeve of basophilc cells LH around infundibulum Neurohypophysis Pars nervosa Nerve fibers and supporting cells Oxytocin and (pituicytes) vasopressin (produced in hypothalamus) Infundibulum Nerve fibers (traveling from hypothalamus to pars nervosa) Pancreas Islet of Langerhans Irregularly arranged cells with Insulin, glucagon many capillaries Follicles: Simple -

Adrenal & Gonadal Hormones Layers of Adrenal Cortex
Adrenal & Gonadal Hormones Topics for today: •Adrenal cortex hormone •Adrenal medulla hormones •Hormone control of organs •Steroid hormone synthesis •Vitamin D3 • Estrogens and Progesterone Layers of adrenal cortex zona glomerulosa zona fasiculata zona reticularis 1 Hormones of adrenal cortex zona glomerulosa zona fasiculata O zona reticularis HO dehydroepiandrosterone Androgens – dehydroepiandrosterone • increased protein synthesis DHEA is weak androgen with • masculinizing effects in almost no effect in male but has female (hypersecretion) masculinizing effects in females Adrenal medulla (interior) • Composed of modified post-synaptic sympathetic neurons • Releases mostly epinephrine. • Has effects similar to those triggered by sympathetic nervous system Adrenal medulla hormones HO HO -CH-CH2 - N-CH3 epinephrine OH H Effects of epinephrine: • causes elevated blood glucose level • stimulates glycolysis & fatty acid use • increases cardiac output & blood pres • shifts blood flow to skeletal muscle • increases rate and depth of respiration 2 Organ responses to Epinephrine Causes glycogen Causes fatty acid release degradation in muscle from adipose tissue fatty glycogen acids trigly- cerides lactate glycerol muscle lactate adipose tissue Causes release of glycogeno lysis glucose from liver glucose ...elevated plasma level liver Hormone action time Epinephrine is in group of fast-acting hormones Fast-acting Hormones Slow-acting Hormones • Norepinephrine • Throxine • Epinephrine • Cortisol • Insulin • Growth hormone • glucagon • Estrogens -
The Sexually Dimorphic Adrenal Cortex: Implications for Adrenal Disease
International Journal of Molecular Sciences Review The Sexually Dimorphic Adrenal Cortex: Implications for Adrenal Disease Rodanthi Lyraki * and Andreas Schedl * Université Côte d’Azur, Inserm, CNRS, Institut de Biologie Valrose, 06108 Nice, France * Correspondence: [email protected] (R.L.); [email protected] (A.S.); Tel.: +33-48915-0730 (R.L.) Abstract: Many adrenocortical diseases are more prevalent in women than in men, but the reasons underlying this sex bias are still unknown. Recent studies involving gonadectomy and sex hormone replacement experiments in mice have shed some light onto the molecular basis of sexual dimorphism in the adrenal cortex. Indeed, it has been shown that gonadal hormones influence many aspects of adrenal physiology, ranging from stem cell-dependent tissue turnover to steroidogenesis and X-zone dynamics. This article reviews current knowledge on adrenal cortex sexual dimorphism and the potential mechanisms underlying sex hormone influence of adrenal homeostasis. Both topics are expected to contribute to personalized and novel therapeutic approaches in the future. Keywords: adrenal cortex; sexual dimorphism; sex hormones; proliferation; adrenocortical carci- noma; Cushing’s syndrome; Addison’s disease; stem cells 1. Introduction Many human diseases of nonreproductive organs display sex bias. While women Citation: Lyraki, R.; Schedl, A. The are more susceptible to autoimmune diseases [1], men show higher incidence and worse Sexually Dimorphic Adrenal Cortex: prognosis in a wide range of cancers [2]. According to systematic phenotypic analysis Implications for Adrenal Disease. Int. of genetically modified mouse lines, sex is a major variable that determines a large pro- J. Mol. Sci. 2021, 22, 4889. https:// portion of mammalian phenotypic traits [3]. -

Anatomy of the Endocrine System
Anatomy of the Endocrine System Meidona N Milla Anatomy Department Faculty of Medicine Sultan Agung Islamic University LEARNING OBJECTIVES • At the end of the lecture, students should be able to describe : The position and structur of the glands of endocrine system:hypothalamus, pituitary, pineal, thyroid, para thyroid, adrenal, pancreas, ovarium, testis Hormones which producing of endocrine system LEARNING OBJECTIVES the structures of hypothalamus related to the pituitary gland. • Describe the blood supply of endocrine system gland . • Describe the blood supply of pituitary gland & the hypophyseal portal system. • The nervous system of endocrine the Endocrine System • The endocrine system is made up of seven different glands that make chemicals called HORMONES. • HORMONES are substances that act as mesenger to control many body function. The endocrine system makes more the 20 mayor hormones that help control: • Growth • Reproduction • Sexsual development • Use and Storage of energy • Responses to physical stress or trauma • Level of fluid salt and sugar in blood GLANDS OF ENDOCRINE SYSTEM • Hypothalamus • Pineal • Pituitary • Thymus • Thyroid • Para thyroid • Adrenal • Pancreas • Gonade ( Ovarium and Testis) HYPOTHALAMUS The hypothalamus is located in the center of the brain. It makes hormones that increase or decrease the release of the hormones made in the pituitary gland it also makes hormones that help to control water balance, sleep, temperature, appetite and blood pressure. Hormones Which produced by hypothalamus Releasing Hormones ( RH) Growth Hormone RH Gonadotrophin RH Thyrotropin RH Prolactin RH Corticotropin RH Release- inhibiting Hormones: Somatostatin Dopamin PITUITARY GLAND (HYPOPHYSIS CEREBRI) It is referred to as the master of endocrine glands. It is a small oval structure 1 cm in diameter. -

The Endocrine System
16 The Endocrine System Lecture Presentation by Lori Garrett © 2018 Pearson Education, Inc. Section 1: Hormones and Intercellular Communication Learning Outcomes 16.1 Describe the similarities between the endocrine and nervous systems and their specific modes of intercellular communication. 16.2 Explain the chemical classification of hormones. 16.3 Identify the organs and tissues of the endocrine system and the key functions of the hormones they secrete. 16.4 Explain the general mechanisms of hormonal action. © 2018 Pearson Education, Inc. Section 1: Hormones and Intercellular Communication Learning Outcomes (continued) 16.5 Describe how the hypothalamus controls endocrine organs. 16.6 Describe the location and structure of the pituitary gland, and identify pituitary hormones and their functions. 16.7 Describe the role of negative feedback in the functional relationship between the hypothalamus and the pituitary gland. © 2018 Pearson Education, Inc. Section 1: Hormones and Intercellular Communication Learning Outcomes (continued) 16.8 Describe the location and structure of the thyroid gland, identify the hormones it produces, and specify the functions of those hormones. 16.9 Describe the location of the parathyroid glands, and identify the functions of the hormone they produce. 16.10 Describe the location, structure, and functions of the adrenal glands, identify the hormones produced, and specify the functions of each hormone. © 2018 Pearson Education, Inc. Section 1: Hormones and Intercellular Communication Learning Outcomes (continued) 16.11 Describe the location and structure of the pancreas, identify the hormones it produces, and specify the functions of those hormones. 16.12 Describe the location of the pineal gland, and identify the functions of the hormone that it produces. -

SSAT ABSITE Review: Endocrine Adrenal, Thyroid, Parathyroid
SSAT ABSITE Review: Endocrine Adrenal, Thyroid, Parathyroid Douglas Cassidy, MD MGH Surgical Education Research and Simulation Fellow @DJCSurgEd https://www.youtube.com/c/surgedvidz 1/22/2020 1 Content Outline • Adrenal: • Parathyroid • Anatomy and Physiology • Anatomy • Incidentalomas • Calcium Homeostasis • Adrenal Cortical Carcinoma • Primary Hyperparathyroidism • Multiple Endocrine Neoplasia • Head and Neck: • Thyroid • Anatomy • Physiology • Neck Dissections • Thyroid Nodules + Ultrasound • Head and Neck Cancers • Thyroid Cancers • Hypo- and Hyper-thyroidism Adrenal Anatomy • Paired RP endocrine glands above superior pole of kidneys • Arterial: • Superior suprarenal from inferior phrenic • Middle suprarenal from abdominal aorta • Inferior suprarenal from renal artery • Venous: • Left adrenal vein drains into the left renal vein • Right adrenal vein drains directly into the IVC Adrenal Incidentalomas: • Evaluation: • Is the mass functioning or non-functioning? • Is the mass benign or malignant? • If malignant, is it primary or secondary? Adrenal Incidentalomas: • Functional Masses: • Adrenal Cortex: • Zona Glomerulosa -- Aldosterone • Zona Fasciculata -- Cortisol • Zona Reticularis -- Androgens • Adrenal Medulla: Catecholamines • Epinephrine / Norepinephrine Aldosteronomas • Function: ↑Na+ absorption and K+ secretion in the distal tubule • ↑H+ excretion in the collecting duct • Labs: ↑Na+, ↓K+, METABOLIC ALKALOSIS • Presentation: uncontrolled / drug- resistant HTN, sxs of low K+ (cramps, weakness) • DDx: • 1°: Adenoma, Hyperplasia,