The Sexually Dimorphic Adrenal Cortex: Implications for Adrenal Disease
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

TEE UNIVERSITY of OKLAHOMA GRADUATE Coiiiege ' A
TEE UNIVERSITY OF OKLAHOMA GRADUATE COIIiEGE ' A COMPARATIVE HISTOLOGICAL AND HISTOCHEMICAL STUDY OF THE ADRENAL GLANDS OF NATIVE RABBITS A THESIS SUBMITTED TO THE GRADUATE FACULTY ±n partial fiolflllment of the requirements for the degree of DOCTOR OF PHILOSOPHY BY I. ERNEST GONZALEZ Oklahoma City, Oklahoma 1955 A COMPARATIVE HISTOLOGICAL AND HISTOCHEMICAL STUDY OF THE ADRENAL GLANDS OF NATIVE RABBITS APEROVED BY THESIS COMMIT' ACKN0WEE33GEMENT The writer wishes to express his profound appreciation and sincere thanks to Dr. Kenneth M. Richter, Department of Anatomy, University of Oklahoma Medical School, for his valuable time, coopera- I _ ition, helpful criticisms, and timely suggestions during the course of j I this investigation; to Dr. Ernest Lachman, Chairman of the Department of Anatomy, for his encouragement and cooperation; to Dr. Garman Daron, Professor of Anatomy, for his many helpful suggestions % and to the University of Oklahoma for a University scholarship. Many other persons have cooperated indirectly in making this investigation possible, and the writer would like to acknowledge also the assistance of Dr. C. Lynn Hayward and Dr. D. Eldon Beck, Depart ment of Zoology, Brigham Young University, for procuring and identify ing many of the native rabbit species; of Mr. Ernest Reiser for his advice during the preparation of the graphic models; and of Mr. Neil Woodward for his assistance with the photomicrographic reproductions. ill TABLE OF CONTENTS Page CHAPTER I 1 Introduction CHAPTER II Materials and MetHods CHAPTER III Observations .............................. 7 Ocbbtona princeps ............»...... ...... 7 Pexicapsular tissue^ capsule, and stroma 7 Vasculature . ......... ............. 8 Innervation............... .... ........ 9 Cortex ........ , . ... ........ ... .. 9 Zona glomerulosa....... ............ 9 Zona fasciculata ............. -

The Morphology, Androgenic Function, Hyperplasia, and Tumors of the Human Ovarian Hilus Cells * William H
THE MORPHOLOGY, ANDROGENIC FUNCTION, HYPERPLASIA, AND TUMORS OF THE HUMAN OVARIAN HILUS CELLS * WILLIAM H. STERNBERG, M.D. (From the Department of Pathology, School of Medicine, Tulane University of Louisiana and the Charity Hospital of Louisiana, New Orleans, La.) The hilus of the human ovary contains nests of cells morphologically identical with testicular Leydig cells, and which, in all probability, pro- duce androgens. Multiple sections through the ovarian hilus and meso- varium will reveal these small nests microscopically in at least 8o per cent of adult ovaries; probably in all adult ovaries if sufficient sections are made. Although they had been noted previously by a number of authors (Aichel,l Bucura,2 and von Winiwarter 3"4) who failed to recog- nize their significance, Berger,5-9 in 1922 and in subsequent years, pre- sented the first sound morphologic studies of the ovarian hilus cells. Nevertheless, there is comparatively little reference to these cells in the American medical literature, and they are not mentioned in stand- ard textbooks of histology, gynecologic pathology, nor in monographs on ovarian tumors (with the exception of Selye's recent "Atlas of Ovarian Tumors"10). The hilus cells are found in clusters along the length of the ovarian hilus and in the adjacent mesovarium. They are, almost without excep- tion, found in contiguity with the nonmyelinated nerves of the hilus, often in intimate relationship to the abundant vascular and lymphatic spaces in this area. Cytologically, a point for point correspondence with the testicular Leydig cells can be established in terms of nuclear and cyto- plasmic detail, lipids, lipochrome pigment, and crystalloids of Reinke. -

I. Introduction B. Adrenal Cortex
I. Introduction Adrenal Glands • suprarenal – they sit on top of the kidneys • each is composed of 2 distinct regions: A. Adrenal Medulla - the inner region - comprises 20% of the gland - secretes epinephrine and norepinephrine - derived from ectoderm B. Adrenal Cortex 1) Zona Glomerulosa (outermost region) - produces mineralocorticoids (aldosterone) • the outer region 2) Zona Fasiculata (middle region) - produces glucocorticoids (cortisol) as well as • comprises 80% of the gland estrogens and androgens • secretes corticosteroids 3) Zona Reticularis (innermost region) • derived from mesoderm - same function as zona fasiculata DHEA – dehydroepiandrosterone • an adrenal androgen in females • responsible for growth of pubic and axillary hair C. Pathologies Associated with Adrenal II. Mineralocorticoids (Aldosterone) Androgen Hypersecretion A. Functions 1.Adrenogenital Syndrome - promotes reabsorption of Na+ and - hypersecretion of androgens or estrogens secretion of K+ from the distal portion of the a) in the adult female: nephron..primary regulator of salt balance and extracellular volume - masculinization (i.e. hirsutism) -Similar (but less important) effect on salt b) in the female embryo: transport in colon, salivary glands, and - female pseudohermaphroditism sweat glands. c) in the adult male: - no effect d) in young boys: - precocious pseudopuberty 1 II. Mineralocorticoids (Aldosterone) C. Pathologies B. Regulation of Secretion 1. Hypersecretion 1. Renin Angiotensin a. primary hyperaldosteronism - Angiotensin II stimulates aldost. secretion - Conn’s syndrome 2. Potassium - usually due to a tumor on the gland + - high levels of K induce aldost. secretion - too much secretion of gland itself 3. ACTH –no direct role b. secondary hyperaldosteronism - default in renin angiotensin system - most common in atherosclerosis of renal arteries C. Pathologies III. Glucocorticoids (Cortisol) 1. -
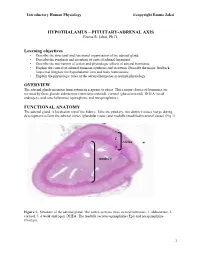
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Gross Anatomy of the Suprarenal Glands
Edited by: Malak Shalfawi, Noor Adnan Gross Anatomy of the suprarenal glands 5/10/2020 Dr. shatarat. The University of Jordan In the sagittal section below, you can see the retroperitoneal space (encircled by a blue line), which contains structures that lie deep on the posterior abdominal wall and are called retroperitoneal structures, they are the kidneys and suprarenal (adrenal) glands. ➔ The adrenal glands are two small triangular structures located retroperitoneally at the upper poles of the kidneys. [notice the black arrow] 5/10/2020 Dr. shatarat. The University of Jordan You can again notice the kidneys (lying on the posterior abdominal wall and covered by fat), The peritoneum and retroperitoneal space. ➔ The adrenal glands are covered with a thick connective tissue capsule from which the trabeculae extend into the parenchyma carrying blood vessels and nerves. **Extra note: all soft structures in the abdomen, such as the spleen, kidneys and suprarenal glands, have hilum into which all blood vessels and nerve supply getting in or out of them. But each one of these soft structures has its specific modifications on its In this section, you can see the hilum. For example, the ureter getting vertebral column and the muscles of out from the kidneys. the posterior abdominal wall (quadratus lumborum and Psoas 5/10/2020 Dr. shatarat. The University of Jordan major) ➔ Adrenal glands are found on the posterior parietal wall, on each side of the vertebral column, at the level of the 11th thoracic rib and lateral to the first lumber vertebra. They are in the upper part of the abdomen, almost near the diaphragm, NOT in the middle and NOT inferior!!!! ➔ They have flattened triangular shape and are embedded in the perirenal fat at the superior poles of the kidneys. -

Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis
Review Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis Athanasios Tselebis 1, Emmanouil Zoumakis 2 and Ioannis Ilias 3,* 1 Department of Psychiatry, Sotiria Hospital, 115 27 Athens, Greece; [email protected] 2 First Department of Pediatrics, National and Kapodistrian University of Athens Medical School, Aghia Sophia Children’s Hospital, 115 27 Athens, Greece; [email protected] 3 Department of Endocrinology, Diabetes and Metabolism, Elena Venizelou Hospital, 115 21 Athens, Greece * Correspondence: [email protected]; Tel.: +30-213-205-1389 Abstract: In this concise review, we present an overview of research on dream recall/affect and of the hypothalamic–pituitary–adrenal (HPA) axis, discussing caveats regarding the action of hormones of the HPA axis (mainly cortisol and its free form, cortisol-binding globulin and glucocorticoid receptors). We present results of studies regarding dream recall/affect and the HPA axis under physiological (such as waking) or pathological conditions (such as in Cushing’s syndrome or stressful situations). Finally, we try to integrate the effect of the current COVID-19 situation with dream recall/affect vis-à-vis the HPA axis. Keywords: dreams; cortisol; stress; memory; sleep; HPA axis 1. Introduction—Dream Recall Almost all humans dream (indeed, there may be 0.5% of people who do not do so) [1]. Dreams are a series of images, thoughts and senses and are considered a specific type Citation: Tselebis, A.; Zoumakis, E.; of experience that occurs in our brain during sleep. During the dream, there is limited Ilias, I. Dream Recall/Affect and the control of the dream content, visual images and memory activation. -

Histogenesis of Suprarenal Glands at Different Gestational Age Groups
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Histogenesis of suprarenal glands at different gestational age groups Ravindra Kumar Boddeti1, Subhadra Devi Velichety2 1Lecturer, 2Professor and Head, Department of Anatomy, Sri Padmavathi Medical College for Women, Sri Venkateswara Institute of Medical Sciences, SVIMS University, Tirupathi, Andhra Pradesh, India Submitted: 22-02-2019 Revised: 10-03-2019 Published: 01-05-2019 ABSTRACT Background: The human foetal suprarenal gland is structurally variant from its adult Access this article online counterpart. The most distinctive features of human foetal suprarenal gland and histologically Website: unique foetal zone, was described first by Elliott and Armour in 1911. After the first trimester, the centrally located foetal zone accounts for most of the foetal adrenal mass. The outer zone http://nepjol.info/index.php/AJMS of the foetal suprarenal gland is called the “definitive zone or neo cortex”; this zone likely DOI: 10.3126/ajms.v10i3.22820 gives rise to the adult adrenal glomerulosa. A third zone called “transitional zone”, lies just E-ISSN: 2091-0576 2467-9100 between the neocortex and foetal zone and is believed to develop into the zona fasciculata. P-ISSN: Aims and Objectives: The current study was designed to study the histogenesis of suprarenal glands at different gestational age groups. Materials and Methods: Twenty-eight formalin preserved dead embryos and foetuses of both sexes, were obtained from the Govt. Maternity Hospital & S.V.Medical College, Tirupati, Andhra Pradesh, India. Specimens were grouped according to their gestational age groups (A,B,C,D) A= 0-12 weeks, B= 13-24 weeks, C= 25-36 weeks and D= more than 36 weeks of gestation. -

Pig Gonads, Adrenal Glands and Brain C
Immunoreactive cytochrome P-45017\g=a\in rat and guinea- pig gonads, adrenal glands and brain C. Le Goascogne1, N. Sanan\l=e'\s1, M. Gou\l=e'\zou1, S. Takemori2, S. Kominami2, E. E. Baulieu1 and P. Robel1 1INSERM U33, Communications Hormonales, and Faculté de Médecine, Université Paris-sud, Lab Hormones F-94275 Bicêtre Cedex France 2 Faculty of Integrated Arts and Sciences, Hiroshima University, Hiroshima 730, Japan Summary. The cytochrome P-45017\g=a\-hydroxylase, 17\ar=r\20lyase (P-45017\g=a\) is the key enzyme responsible for the biosynthesis of androgens in steroidogenic organs. Its cellular localization has been examined with an immunohistochemical technique. In immature rat ovary, P-45017\g=a\was first detected in sparse interstitial cells on postnatal Day 8. The number of immunoreactive interstitial cells increased thereafter and the intensity of P-45017\g=a\staining in these cells was highest at 3 weeks of age. The intensity of staining then started to decline and was very faint at Day 35. From 6 weeks on, the distribution of immunoreactive P-45017\g=a\was of the adult type: it was detected exclusively in the thecal cells of the large antral, preovulatory, follicles. P-45017\g=a\was not detectable during pregnancy except on the day of parturition, when thecal cells were transiently immunoreactive. The staining had vanished 24 h after delivery. Human chorionic gonadotrophin (hCG), injected into immature females on Days 24 to 26, induced P-45017\g=a\prematurely in thecal cells. When injected on Days 12 to 14 of pregnancy, hCG also induced P-45017\g=a\in the thecal cells surrounding the largest follicles, whereas the interstitial and luteal cells were not immunostained. -

Adrenal Gland Hormones
CHAPTER 8 Adrenal Gland Hormones Devra K. Dang, PharmD, BCPS, CDE, FNAP | Trinh Pham, PharmD, BCOP | Jennifer J. Lee, PharmD, BCPS, CDE LEARNING OBJECTIVES KEY TERMS AND DEFINITIONS After completing this chapter, you should be able to ACTH (adrenocorticotropic hormone) — a hormone produced 1. Identify the hormones produced by the adrenal glands by the pituitary gland that stimulates 2. Describe the functions of mineralocorticoids and glucocorticoids in the body the adrenal cortex to produce glucocorticoids, mineralocorticoids, 3. Recognize the signs and symptoms of adrenal insuffi ciency and androgens. PART 4. Describe the pharmacological treatment of patients with acute and chronic adrenal Addison ’ s disease — a disorder insuffi ciency in which the adrenal glands do not produce enough steroid hormones. 3 5. Recognize the signs and symptoms of Cushing ’ s syndrome and the result of too Adenoma — a benign much cortisol (noncancerous) tumor of glandular 6. Describe the pharmacologic and nonpharmacologic management of patients with origin. Cushing ’ s syndrome Adrenal insuffi ciency — a term 7. List management strategies for administration of glucocorticoid and mineralocorti- referring to a defi ciency in the levels of adrenal hormones. coid therapy to avoid development of adrenal disorders Aldosterone — the hormone produced by the adrenal glands that regulates the balance of sodium, he adrenal glands are an integral part of the endocrine system, secreting water, and potassium concentrations in the body. T hormones that act throughout the body to regulate functions and promote Corticotropin-releasing homeostasis. In addition to the neurotransmitters epinephrine and norepineph- hormone (CRH) — a hormone rine, the corticosteroids secreted by the adrenal glands are vital to a wide released by the hypothalamus that variety of physiological processes. -

PKA Signaling Drives Reticularis Differentiation and Sexually Dimorphic Adrenal Cortex Renewal
PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal Typhanie Dumontet, … , Pierre Val, Antoine Martinez JCI Insight. 2018;3(2):e98394. https://doi.org/10.1172/jci.insight.98394. Research Article Development Endocrinology The adrenal cortex undergoes remodeling during fetal and postnatal life. How zona reticularis emerges in the postnatal gland to support adrenarche, a process whereby higher primates increase prepubertal androgen secretion, is unknown. Using cell-fate mapping and gene deletion studies in mice, we show that activation of PKA has no effect on the fetal cortex, while it accelerates regeneration of the adult cortex, triggers zona fasciculata differentiation that is subsequently converted into a functional reticularis-like zone, and drives hypersecretion syndromes. Remarkably, PKA effects are influenced by sex. Indeed, testicular androgens increase WNT signaling that antagonizes PKA, leading to slower adrenocortical cell turnover and delayed phenotype whereas gonadectomy sensitizes males to hypercorticism and reticularis-like formation. Thus, reticularis results from ultimate centripetal conversion of adult cortex under the combined effects of PKA and cell turnover that dictate organ size. We show that PKA-induced progenitor recruitment is sexually dimorphic and may provide a paradigm for overrepresentation of women in adrenal diseases. Find the latest version: https://jci.me/98394/pdf RESEARCH ARTICLE PKA signaling drives reticularis differentiation and sexually dimorphic adrenal cortex renewal Typhanie Dumontet,1 Isabelle Sahut-Barnola,1 Amandine Septier,1 Nathanaëlle Montanier,1 Ingrid Plotton,2 Florence Roucher-Boulez,2 Véronique Ducros,3 Anne-Marie Lefrançois-Martinez,1 Jean-Christophe Pointud,1 Mohamad Zubair,4 Ken-Ichirou Morohashi,4 David T. Breault,5,6 Pierre Val,1 and Antoine Martinez1 1GReD, Université Clermont Auvergne, CNRS, INSERM, Clermont-Ferrand, France. -

Hypothalamushypothalamus -- Pituitarypituitary -- Adrenaladrenal Glandsglands
HypothalamusHypothalamus -- pituitarypituitary -- adrenaladrenal glandsglands Magdalena Gibas-Dorna MD, PhD Dept. of Physiology University of Medical Sciences Poznań, Poland Hypothalamus - general director of the hormone system. At every moment, the hypothalamus analyses messages coming from: the brain and different regions of the body. Homeostatic functions of hypothalamus include maintaining a stable body temperature, controlling food intake, controlling blood pressure, ensuring a fluid balance, and even proper sleep patterns. Cell bodies of neurons that produce releasing/inhibiting hormones Hypothalamus HypothalamusHypothalamus releases Arterial flow Primary capillaries in median eminence hormones at Long Releasing Portal hormones Anterior veins median eminence pituitary hormone Releasing/ inhibiting hormones and sends to anterior pituitary ANTERIOR PITUITARY via portalportal veinvein. Secretory cells that produce anterior pituitary hormones Anterior pituitary hormones Venous outflow Gonadotropic Thyroid- Proactin hormones stimulating ACTH Growth (FSH and LH) hormone hormone ControlControl ofof pituitarypituitary hormonehormone secretionsecretion byby hypothalamushypothalamus • Secretion by the anterioranterior pituitarypituitary is controlled by hormones called hypothalamic releasing hormones and inhibitory hormones conducted to the anterior pituitary through hypothalamichypothalamic -- hypophysialhypophysial portalportal vesselsvessels .. • PosteriorPosterior pituitarypituitary secrets two hormones, which are synthesized within cell