Adrenal Cortical Tumors, Pheochromocytomas and Paragangliomas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Adrenal Cancer Causes, Risk Factors, and Prevention Adrenal
cancer.org | 1.800.227.2345 Adrenal Cancer Causes, Risk Factors, and Prevention Risk Factors A risk factor is anything that affects your chance of getting a disease such as cancer. Learn more about the risk factors for adrenal cancer. ● Adrenal Cancer Risk Factors ● What Causes Adrenal Cancer? Prevention Since there are no known preventable risk factors for this cancer, it is not possible to prevent this disease. Adrenal Cancer Risk Factors A risk factor is anything that changes your chance of getting a disease such as cancer. Different cancers have different risk factors. Some risk factors, like smoking, can be changed. Others, like a person’s age or family history, can’t be changed. Scientists have found few risk factors that make a person more likely to develop adrenal cancer. Even if a patient does have one or more risk factors for adrenal cancer, it is impossible to know for sure how much that risk factor contributed to causing the cancer. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 But having a risk factor, or even several, does not mean that you will get the disease. Many people with risk factors never develop adrenal cancer, while others with this disease may have few or no known risk factors. Genetic syndromes The majority of adrenal cortex cancers are not inherited (sporadic), but some (up to 15%) are caused by a genetic defect. This is more common in adrenal cancers in children. Li-Fraumeni syndrome The Li-Fraumeni syndrome is a rare condition that is most often caused by a defect in the TP53 gene. -

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

TEE UNIVERSITY of OKLAHOMA GRADUATE Coiiiege ' A
TEE UNIVERSITY OF OKLAHOMA GRADUATE COIIiEGE ' A COMPARATIVE HISTOLOGICAL AND HISTOCHEMICAL STUDY OF THE ADRENAL GLANDS OF NATIVE RABBITS A THESIS SUBMITTED TO THE GRADUATE FACULTY ±n partial fiolflllment of the requirements for the degree of DOCTOR OF PHILOSOPHY BY I. ERNEST GONZALEZ Oklahoma City, Oklahoma 1955 A COMPARATIVE HISTOLOGICAL AND HISTOCHEMICAL STUDY OF THE ADRENAL GLANDS OF NATIVE RABBITS APEROVED BY THESIS COMMIT' ACKN0WEE33GEMENT The writer wishes to express his profound appreciation and sincere thanks to Dr. Kenneth M. Richter, Department of Anatomy, University of Oklahoma Medical School, for his valuable time, coopera- I _ ition, helpful criticisms, and timely suggestions during the course of j I this investigation; to Dr. Ernest Lachman, Chairman of the Department of Anatomy, for his encouragement and cooperation; to Dr. Garman Daron, Professor of Anatomy, for his many helpful suggestions % and to the University of Oklahoma for a University scholarship. Many other persons have cooperated indirectly in making this investigation possible, and the writer would like to acknowledge also the assistance of Dr. C. Lynn Hayward and Dr. D. Eldon Beck, Depart ment of Zoology, Brigham Young University, for procuring and identify ing many of the native rabbit species; of Mr. Ernest Reiser for his advice during the preparation of the graphic models; and of Mr. Neil Woodward for his assistance with the photomicrographic reproductions. ill TABLE OF CONTENTS Page CHAPTER I 1 Introduction CHAPTER II Materials and MetHods CHAPTER III Observations .............................. 7 Ocbbtona princeps ............»...... ...... 7 Pexicapsular tissue^ capsule, and stroma 7 Vasculature . ......... ............. 8 Innervation............... .... ........ 9 Cortex ........ , . ... ........ ... .. 9 Zona glomerulosa....... ............ 9 Zona fasciculata ............. -

The Morphology, Androgenic Function, Hyperplasia, and Tumors of the Human Ovarian Hilus Cells * William H
THE MORPHOLOGY, ANDROGENIC FUNCTION, HYPERPLASIA, AND TUMORS OF THE HUMAN OVARIAN HILUS CELLS * WILLIAM H. STERNBERG, M.D. (From the Department of Pathology, School of Medicine, Tulane University of Louisiana and the Charity Hospital of Louisiana, New Orleans, La.) The hilus of the human ovary contains nests of cells morphologically identical with testicular Leydig cells, and which, in all probability, pro- duce androgens. Multiple sections through the ovarian hilus and meso- varium will reveal these small nests microscopically in at least 8o per cent of adult ovaries; probably in all adult ovaries if sufficient sections are made. Although they had been noted previously by a number of authors (Aichel,l Bucura,2 and von Winiwarter 3"4) who failed to recog- nize their significance, Berger,5-9 in 1922 and in subsequent years, pre- sented the first sound morphologic studies of the ovarian hilus cells. Nevertheless, there is comparatively little reference to these cells in the American medical literature, and they are not mentioned in stand- ard textbooks of histology, gynecologic pathology, nor in monographs on ovarian tumors (with the exception of Selye's recent "Atlas of Ovarian Tumors"10). The hilus cells are found in clusters along the length of the ovarian hilus and in the adjacent mesovarium. They are, almost without excep- tion, found in contiguity with the nonmyelinated nerves of the hilus, often in intimate relationship to the abundant vascular and lymphatic spaces in this area. Cytologically, a point for point correspondence with the testicular Leydig cells can be established in terms of nuclear and cyto- plasmic detail, lipids, lipochrome pigment, and crystalloids of Reinke. -

Endocrine Pathology (537-577)
LABORATORY INVESTIGATION THE BASIC AND TRANSLATIONAL PATHOLOGY RESEARCH JOURNAL LI VOLUME 99 | SUPPLEMENT 1 | MARCH 2019 2019 ABSTRACTS ENDOCRINE PATHOLOGY (537-577) MARCH 16-21, 2019 PLATF OR M & 2 01 9 ABSTRACTS P OSTER PRESENTATI ONS EDUCATI ON C O M MITTEE Jason L. Hornick , C h air Ja mes R. Cook R h o n d a K. Y a nti s s, Chair, Abstract Revie w Board S ar a h M. Dr y and Assign ment Co m mittee Willi a m C. F a q ui n Laura W. La mps , Chair, C ME Subco m mittee C ar ol F. F ar v er St e v e n D. Billi n g s , Interactive Microscopy Subco m mittee Y uri F e d ori w Shree G. Shar ma , Infor matics Subco m mittee Meera R. Ha meed R aj a R. S e et h al a , Short Course Coordinator Mi c h ell e S. Hir s c h Il a n W ei nr e b , Subco m mittee for Unique Live Course Offerings Laksh mi Priya Kunju D a vi d B. K a mi n s k y ( Ex- Of ici o) A n n a M ari e M ulli g a n Aleodor ( Doru) Andea Ri s h P ai Zubair Baloch Vi nita Parkas h Olca Bast urk A nil P ar w a ni Gregory R. Bean , Pat h ol o gist-i n- Trai ni n g D e e p a P atil D a ni el J. -

I. Introduction B. Adrenal Cortex
I. Introduction Adrenal Glands • suprarenal – they sit on top of the kidneys • each is composed of 2 distinct regions: A. Adrenal Medulla - the inner region - comprises 20% of the gland - secretes epinephrine and norepinephrine - derived from ectoderm B. Adrenal Cortex 1) Zona Glomerulosa (outermost region) - produces mineralocorticoids (aldosterone) • the outer region 2) Zona Fasiculata (middle region) - produces glucocorticoids (cortisol) as well as • comprises 80% of the gland estrogens and androgens • secretes corticosteroids 3) Zona Reticularis (innermost region) • derived from mesoderm - same function as zona fasiculata DHEA – dehydroepiandrosterone • an adrenal androgen in females • responsible for growth of pubic and axillary hair C. Pathologies Associated with Adrenal II. Mineralocorticoids (Aldosterone) Androgen Hypersecretion A. Functions 1.Adrenogenital Syndrome - promotes reabsorption of Na+ and - hypersecretion of androgens or estrogens secretion of K+ from the distal portion of the a) in the adult female: nephron..primary regulator of salt balance and extracellular volume - masculinization (i.e. hirsutism) -Similar (but less important) effect on salt b) in the female embryo: transport in colon, salivary glands, and - female pseudohermaphroditism sweat glands. c) in the adult male: - no effect d) in young boys: - precocious pseudopuberty 1 II. Mineralocorticoids (Aldosterone) C. Pathologies B. Regulation of Secretion 1. Hypersecretion 1. Renin Angiotensin a. primary hyperaldosteronism - Angiotensin II stimulates aldost. secretion - Conn’s syndrome 2. Potassium - usually due to a tumor on the gland + - high levels of K induce aldost. secretion - too much secretion of gland itself 3. ACTH –no direct role b. secondary hyperaldosteronism - default in renin angiotensin system - most common in atherosclerosis of renal arteries C. Pathologies III. Glucocorticoids (Cortisol) 1. -
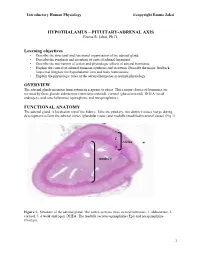
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

Adrenal Neuroblastoma Mimicking Pheochromocytoma in an Adult With
Khalayleh et al. Int Arch Endocrinol Clin Res 2017, 3:008 Volume 3 | Issue 1 International Archives of Endocrinology Clinical Research Case Report : Open Access Adrenal Neuroblastoma Mimicking Pheochromocytoma in an Adult with Neurofibromatosis Type 1 Harbi Khalayleh1, Hilla Knobler2, Vitaly Medvedovsky2, Edit Feldberg3, Judith Diment3, Lena Pinkas4, Guennadi Kouniavsky1 and Taiba Zornitzki2* 1Department of Surgery, Hebrew University Medical School of Jerusalem, Israel 2Endocrinology, Diabetes and Metabolism Institute, Kaplan Medical Center, Hebrew University Medical School of Jerusalem, Israel 3Pathology Institute, Kaplan Medical Center, Israel 4Nuclear Medicine Institute, Kaplan Medical Center, Israel *Corresponding author: Taiba Zornitzki, MD, Endocrinology, Diabetes and Metabolism Institute, Kaplan Medical Center, Hebrew University Medical School of Jerusalem, Bilu 1, 76100 Rehovot, Israel, Tel: +972-894- 41315, Fax: +972-8 944-1912, E-mail: [email protected] Context 2. This is the first reported case of an adrenal neuroblastoma occurring in an adult patient with NF1 presenting as a large Neurofibromatosis type 1 (NF1) is a genetic disorder asso- adrenal mass with increased catecholamine levels mimicking ciated with an increased risk of malignant disorders. Adrenal a pheochromocytoma. neuroblastoma is considered an extremely rare tumor in adults and was not previously described in association with NF1. 3. This case demonstrates the clinical overlap between pheo- Case description: A 42-year-old normotensive woman with chromocytoma and neuroblastoma. typical signs of NF1 underwent evaluation for abdominal pain, Keywords and a large 14 × 10 × 16 cm left adrenal mass displacing the Adrenal neuroblastoma, Neurofibromatosis type 1, Pheo- spleen, pancreas and colon was found. An initial diagnosis of chromocytoma, Neural crest-derived tumors pheochromocytoma was done based on the known strong association between pheochromocytoma, NF1 and increased catecholamine levels. -

Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis
Review Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis Athanasios Tselebis 1, Emmanouil Zoumakis 2 and Ioannis Ilias 3,* 1 Department of Psychiatry, Sotiria Hospital, 115 27 Athens, Greece; [email protected] 2 First Department of Pediatrics, National and Kapodistrian University of Athens Medical School, Aghia Sophia Children’s Hospital, 115 27 Athens, Greece; [email protected] 3 Department of Endocrinology, Diabetes and Metabolism, Elena Venizelou Hospital, 115 21 Athens, Greece * Correspondence: [email protected]; Tel.: +30-213-205-1389 Abstract: In this concise review, we present an overview of research on dream recall/affect and of the hypothalamic–pituitary–adrenal (HPA) axis, discussing caveats regarding the action of hormones of the HPA axis (mainly cortisol and its free form, cortisol-binding globulin and glucocorticoid receptors). We present results of studies regarding dream recall/affect and the HPA axis under physiological (such as waking) or pathological conditions (such as in Cushing’s syndrome or stressful situations). Finally, we try to integrate the effect of the current COVID-19 situation with dream recall/affect vis-à-vis the HPA axis. Keywords: dreams; cortisol; stress; memory; sleep; HPA axis 1. Introduction—Dream Recall Almost all humans dream (indeed, there may be 0.5% of people who do not do so) [1]. Dreams are a series of images, thoughts and senses and are considered a specific type Citation: Tselebis, A.; Zoumakis, E.; of experience that occurs in our brain during sleep. During the dream, there is limited Ilias, I. Dream Recall/Affect and the control of the dream content, visual images and memory activation. -

Limited Diagnostic Utility of Chromogranin a Measurements in Workup of Neuroendocrine Tumors
diagnostics Article Limited Diagnostic Utility of Chromogranin A Measurements in Workup of Neuroendocrine Tumors Jonas Baekdal 1,2,*, Jesper Krogh 1,2, Marianne Klose 1,2, Pernille Holmager 1,2, Seppo W. Langer 1,3, Peter Oturai 1,4,5, Andreas Kjaer 1,4,5 , Birgitte Federspiel 1,6, Linda Hilsted 1,7, Jens F. Rehfeld 1,7, Ulrich Knigge 1,2,8 and Mikkel Andreassen 1,2 1 ENETS Neuroendocrine Tumor Centre of Excellence, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark; [email protected] (J.K.); [email protected] (M.K.); [email protected] (P.H.); [email protected] (S.W.L.); [email protected] (P.O.); [email protected] (A.K.); [email protected] (B.F.); [email protected] (L.H.); [email protected] (J.F.R.); [email protected] (U.K.); [email protected] (M.A.) 2 Department of Endocrinology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 3 Department of Oncology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 4 Department of Clinical Physiology, Nuclear Medicine & PET and Cluster for Molecular Imaging, Copenhagen University Hospital, 2100 Copenhagen, Denmark 5 Department of Biomedical Sciences, Rigshospitalet and University of Copenhagen, 2100 Copenhagen, Denmark 6 Department of Pathology, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 7 Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark 8 Department of Surgery and Transplantation, Rigshospitalet, Copenhagen University Hospital, 2100 Copenhagen, Denmark * Correspondence: [email protected]; Tel.: +45-6013-4687 Received: 11 October 2020; Accepted: 28 October 2020; Published: 29 October 2020 Abstract: Background: Plasma chromogranin A (CgA) is related to tumor burden and recommended in the follow-up of patients diagnosed with neuroendocrine tumors (NETs). -

Small-Cell Neuroendocrine Tumors: Cell State Trumps the Oncogenic Driver Matthew G
Published OnlineFirst January 26, 2018; DOI: 10.1158/1078-0432.CCR-17-3646 CCR Translations Clinical Cancer Research Small-Cell Neuroendocrine Tumors: Cell State Trumps the Oncogenic Driver Matthew G. Oser1,2 and Pasi A. Janne€ 1,2,3 Small-cell neuroendocrine cancers often originate in the lung SCCB and SCLC share common genetic driver mutations. but can also arise in the bladder or prostate. Phenotypically, Clin Cancer Res; 24(8); 1775–6. Ó2018 AACR. small-cell carcinoma of the bladder (SCCB) shares many simi- See related article by Chang et al., p. 1965 larities with small-cell lung cancer (SCLC). It is unknown whether In this issue of Clinical Cancer Research, Chang and colleagues ponent, suggesting that RB1 and TP53 loss occurs after the initial (1) perform DNA sequencing to characterize the mutational development of the urothelial carcinoma and is required for signature of small-cell carcinoma of the bladder (SCCB). They transdifferentiation from urothelial cancer to SCCB. This is rem- find that both SCCB and small-cell lung cancer (SCLC) harbor iniscent of a similar phenomenon observed in two other tumors near universal loss-of-function mutations in RB1 and TP53.In types: (i) EGFR-mutant lung cancer and (ii) castration-resistant contrast to the smoking mutational signature found in SCLC, prostate cancer, where RB1 and TP53 loss is necessary for the SCCB has an APOBEC mutational signature, a signature also transdifferentiation from an adenocarcinoma to a small-cell neu- found in urothelial carcinoma. Furthermore, they show that SCCB roendocrine tumor. EGFR-mutant lung cancers acquire RB1 loss as and urothelial carcinoma share many common mutations that are a mechanism of resistance to EGFR tyrosine kinase inhibitors (3), distinct from mutations found in SCLC, suggesting that SCCB and castration-resistant prostate cancers acquire RB1 and TP53 may arise from a preexisting urothelial cancer. -

Histogenesis of Suprarenal Glands at Different Gestational Age Groups
ORIGINAL ARTICLE ASIAN JOURNAL OF MEDICAL SCIENCES Histogenesis of suprarenal glands at different gestational age groups Ravindra Kumar Boddeti1, Subhadra Devi Velichety2 1Lecturer, 2Professor and Head, Department of Anatomy, Sri Padmavathi Medical College for Women, Sri Venkateswara Institute of Medical Sciences, SVIMS University, Tirupathi, Andhra Pradesh, India Submitted: 22-02-2019 Revised: 10-03-2019 Published: 01-05-2019 ABSTRACT Background: The human foetal suprarenal gland is structurally variant from its adult Access this article online counterpart. The most distinctive features of human foetal suprarenal gland and histologically Website: unique foetal zone, was described first by Elliott and Armour in 1911. After the first trimester, the centrally located foetal zone accounts for most of the foetal adrenal mass. The outer zone http://nepjol.info/index.php/AJMS of the foetal suprarenal gland is called the “definitive zone or neo cortex”; this zone likely DOI: 10.3126/ajms.v10i3.22820 gives rise to the adult adrenal glomerulosa. A third zone called “transitional zone”, lies just E-ISSN: 2091-0576 2467-9100 between the neocortex and foetal zone and is believed to develop into the zona fasciculata. P-ISSN: Aims and Objectives: The current study was designed to study the histogenesis of suprarenal glands at different gestational age groups. Materials and Methods: Twenty-eight formalin preserved dead embryos and foetuses of both sexes, were obtained from the Govt. Maternity Hospital & S.V.Medical College, Tirupati, Andhra Pradesh, India. Specimens were grouped according to their gestational age groups (A,B,C,D) A= 0-12 weeks, B= 13-24 weeks, C= 25-36 weeks and D= more than 36 weeks of gestation.