Endocrine System 4: Adrenal Glands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
2 Surgical Anatomy
2 Surgical Anatomy Nancy Dugal Perrier, Michael Sean Boger contents 2.2 Morphology 2.1 Introduction . 7 The paired retroperitoneal adrenal glands are found 2.2 Morphology ...7 in the middle of the abdominal cavity, residing on 2.3 Relationship of the Adrenal Glands to the Kidneys ...10 the superior medial aspect of the upper pole of each 2.4 Blood Supply, Innervation, and Lymphatics ...10 kidney (Fig. 1). However, this location may vary 2.4.1 Arterial . 10 depending on the depth of adipose tissue.By means of 2.4.2 Venous ...10 pararenal fat and perirenal fascia,the adrenals contact 2.4.3 Innervation ...11 the superior portion of the abdominal wall. These 2.4.4 Lymphatic . 11 structures separate the adrenals from the pleural re- 2.5 Left Adrenal Gland Relationships ...11 flection, ribs, and the subcostal, sacrospinalis, and 2.6 Right Adrenal Gland Relationships ...14 latissimus dorsi muscles [2].Posteriorly,the glands lie 2.7 Summary ...17 References . 17 near the diaphragmatic crus and arcuate ligament [10]. Laterally, the right adrenal resides in front of the 12th rib and the left gland is in front of the 11th and 12th ribs [2]. Each adrenal gland weighs approximately 2.1 Introduction Liver Adrenal gland The small paired adrenal glands have a grand history. Eustachius published the first anatomical drawings of the adrenal glands in the mid-sixteenth century [17].In 1586, Piccolomineus and Baunin named them the suprarenal glands. Nearly two-and-a-half centuries later, Cuvier described the anatomical division of each gland into the cortex and medulla. -

Anatomical Variations in the Arterial Supply of the Suprarenal Gland. Int J Health Sci Res
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Anatomical Variations in the Arterial Supply of the Suprarenal Gland Sushma R.K1, Mahesh Dhoot2, Hemant Ashish Harode2, Antony Sylvan D’Souza3, Mamatha H4 1Lecturer, 2Postgraduate, 3Professor & Head, 4Assistant Professor; Department of Anatomy, Kasturba Medical College, Manipal University, Manipal-576104, Karnataka, India. Corresponding Author: Mamatha H Received: 29/03//2014 Revised: 17/04/2014 Accepted: 21/04/2014 ABSTRACT Introduction: Suprarenal gland is normally supplied by superior, middle and inferior suprarenal arteries which are the branches of inferior phrenic, abdominal aorta and renal artery respectively. However the arterial supply of the suprarenal gland may show variations. Therefore a study was conducted to find the variations in the arterial supply of Suprarenal Gland. Materials and methods: 20 Formalin fixed cadavers, were dissected bilaterally in the department of Anatomy, Kasturba Medical College, Manipal to study the arterial supply of the suprarenal gland, which were photographed and different variations were noted. Results: Out of 20 cadavers variations were observed in five cases in the arterial pattern of supra renal gland. We found that in one cadaver superior supra renal artery on the left side was arising directly from the coeliac trunk. Another variation was observed on the right side ina cadaver that inferior and middle suprarenal arteries were arising from accessory renal artery and on the right side it gave another small branch to the gland. Conclusion: Variations in the arterial pattern of suprarenal gland are significant for radiological and surgical interventions. KEY WORDS: Suprarenal gland, suprarenal artery, renal artery, abdominal aorta, inferior phrenic artery INTRODUCTION accessory renal arteries (ARA). -

Expression Pattern of Delta-Like 1 Homolog in Developing Sympathetic Neurons and Chromaffin Cells
Published in "Gene Expression Patterns 30: 49–54, 2018" which should be cited to refer to this work. Expression pattern of delta-like 1 homolog in developing sympathetic neurons and chromaffin cells ∗ Tehani El Faitwria,b, Katrin Hubera,c, a Institute of Anatomy & Cell Biology, Albert-Ludwigs-University Freiburg, Albert-Str. 17, 79104, Freiburg, Germany b Department of Histology and Anatomy, Faculty of Medicine, Benghazi University, Benghazi, Libya c Department of Medicine, University of Fribourg, Route Albert-Gockel 1, 1700, Fribourg, Switzerland ABSTRACT Keywords: Delta-like 1 homolog (DLK1) is a member of the epidermal growth factor (EGF)-like family and an atypical notch Sympathetic neurons ligand that is widely expressed during early mammalian development with putative functions in the regulation Chromaffin cells of cell differentiation and proliferation. During later stages of development, DLK1 is downregulated and becomes DLK1 increasingly restricted to specific cell types, including several types of endocrine cells. DLK1 has been linked to Adrenal gland various tumors and associated with tumor stem cell features. Sympathoadrenal precursors are neural crest de- Organ of Zuckerkandl rived cells that give rise to either sympathetic neurons of the autonomic nervous system or the endocrine Development ffi Neural crest chroma n cells located in the adrenal medulla or extraadrenal positions. As these cells are the putative cellular Phox2B origin of neuroblastoma, one of the most common malignant tumors in early childhood, their molecular char- acterization is of high clinical importance. In this study we have examined the precise spatiotemporal expression of DLK1 in developing sympathoadrenal cells. We show that DLK1 mRNA is highly expressed in early sympa- thetic neuron progenitors and that its expression depends on the presence of Phox2B. -

Adrenal Metastasis from an Esophageal Squamous Cell Carcinoma - a Case Report and Review of Literature
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 15, Issue 10 Ver. VII (October. 2016), PP 20-22 www.iosrjournals.org Adrenal Metastasis from an Esophageal Squamous Cell Carcinoma - A Case Report and Review of Literature Prof. Subbiah Shanmugam MS Mch1, Dr Sujay Susikar MS Mch2, Dr. H Prasanna Srinivasa Rao Mch Post Graduate2 1,2Department Of Surgical Oncology, Centre For Oncology, Government Royapettah Hospital & Kilpauk Medical College, Chennai, India Abstract: Adrenal metastasis from esophageal carcinoma is quite uncommon. The identification of adrenal metastasis and their differentiation from incidentally detected benign adrenal tumors is challenging especially when functional imaging facilities are unavailable. Here we present a case report of a 43 year old male presenting with adrenal metastasis from an esophageal squamous cell carcinoma. The use of minimally invasive surgery to confirm the metastatic nature of disease in a resource limited setup has been described. Keywords: adrenal metastasis, adrenalectomy, esophageal squamous cell carcinoma I. Introduction Adrenal metastases have been reported in various malignancies; most commonly from cancers of lung, breast but uncommonly from esophageal primary. The diagnostic difficulties in the identification of adrenal secondaries are due to the small size of the lesion, difficulty in differentiating benign from malignant adrenal lesions based on computed tomography findings alone and the anatomical position of adrenal making it difficult to target for biopsy under image guidance. The functional scans (PET CT) not only reliably differentiate metastatic adrenal lesions, but also light up other areas of metastasis. Such information is definitely needed before deciding on the intent of treatment and the surgery for the primary lesion. -

A Study on the Variations of Arterial Supply to Adrenal Gland
K Naga Vidya Lakshmi and Mahesh Dhoot / International Journal of Biomedical and Advance Research 2016; 7(8): 373-375. 373 International Journal of Biomedical and Advance Research ISSN: 2229-3809 (Online); 2455-0558 (Print) Journal DOI: 10.7439/ijbar CODEN: IJBABN Original Research Article A study on the variations of arterial supply to adrenal gland K Naga Vidya Lakshmi* and Mahesh Dhoot Department of Anatomy, RKDF Medical College Hospital & Research Centre, Bhopal, India *Correspondence Info: Dr. K Naga Vidya Lakshmi, Room No: 208, Staff Quarters, RKDF Medical College Campus, Jatkhedi, Bhopal, India E-mail: [email protected] Abstract Introduction: Adrenal glands are richly vascular and get their arterial supply by means of three arteries namely superior, middle, inferior suprarenal arteries. The inferior phrenic artery gives off the superior branch, while middle branch arises directly from the abdominal aorta, and the inferior suprarenal branch is given off by the renal artery. Materials And Methods: The study was conducted in 15 formalin fixed cadavers obtained from the department of Anatomy and are carefully dissected to observe the arterial supply of both right and left adrenal glands. Observations: It has been noted that variations were observed in four cases out of 30 adrenal glands, two cases showed variations on right side and in two cases variation is seen on left side. First case on the right side, both MSA and ISA are given off by ARA. In the second case on right side IPA is given off by RA and from the junction of these two arteries MSA was given. In third case on left side MSA is given by the coeliac trunk and ISA is from ARA, in fourth case on left side ISA is originated from ARA. -

I. Introduction B. Adrenal Cortex
I. Introduction Adrenal Glands • suprarenal – they sit on top of the kidneys • each is composed of 2 distinct regions: A. Adrenal Medulla - the inner region - comprises 20% of the gland - secretes epinephrine and norepinephrine - derived from ectoderm B. Adrenal Cortex 1) Zona Glomerulosa (outermost region) - produces mineralocorticoids (aldosterone) • the outer region 2) Zona Fasiculata (middle region) - produces glucocorticoids (cortisol) as well as • comprises 80% of the gland estrogens and androgens • secretes corticosteroids 3) Zona Reticularis (innermost region) • derived from mesoderm - same function as zona fasiculata DHEA – dehydroepiandrosterone • an adrenal androgen in females • responsible for growth of pubic and axillary hair C. Pathologies Associated with Adrenal II. Mineralocorticoids (Aldosterone) Androgen Hypersecretion A. Functions 1.Adrenogenital Syndrome - promotes reabsorption of Na+ and - hypersecretion of androgens or estrogens secretion of K+ from the distal portion of the a) in the adult female: nephron..primary regulator of salt balance and extracellular volume - masculinization (i.e. hirsutism) -Similar (but less important) effect on salt b) in the female embryo: transport in colon, salivary glands, and - female pseudohermaphroditism sweat glands. c) in the adult male: - no effect d) in young boys: - precocious pseudopuberty 1 II. Mineralocorticoids (Aldosterone) C. Pathologies B. Regulation of Secretion 1. Hypersecretion 1. Renin Angiotensin a. primary hyperaldosteronism - Angiotensin II stimulates aldost. secretion - Conn’s syndrome 2. Potassium - usually due to a tumor on the gland + - high levels of K induce aldost. secretion - too much secretion of gland itself 3. ACTH –no direct role b. secondary hyperaldosteronism - default in renin angiotensin system - most common in atherosclerosis of renal arteries C. Pathologies III. Glucocorticoids (Cortisol) 1. -
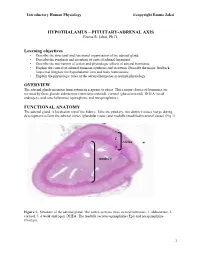
HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Learning Objectives OVERVIEW FUNCTIONAL ANATOMY
Introductory Human Physiology ©copyright Emma Jakoi HYPOTHALAMUS – PITUITARY-ADRENAL AXIS Emma R. Jakoi, Ph.D. Learning objectives • Describe the structural and functional organization of the adrenal gland. • Describe the synthesis and secretion of cortical adrenal hormones. • Describe the mechanism of action and physiologic effects of adrenal hormones. • Explain the control of adrenal hormone synthesis and secretion. Describe the major feedback loops that integrate the hypothalamic axis and body homeostasis. • Explain the physiologic roles of the adrenal hormones in normal physiology. OVERVIEW The adrenal glands maintain homeostasis in response to stress. Three major classes of hormones are secreted by these glands: aldosterone (mineralocorticoid), cortisol (glucocorticoid), DHEA (weak androgen), and catecholamines (epinephrine and norepinephrine). FUNCTIONAL ANATOMY The adrenal gland is located on top of the kidney. Like the pituitary, two distinct tissues merge during development to form the adrenal cortex (glandular tissue) and medulla (modified neuronal tissue) (Fig 1). 1 2 cortex 3 medulla Figure 1. Structure of the adrenal gland. The cortex secretes three steroid hormones: 1. aldosterone, 2. cortisol, 3. a weak androgen, DHEA. The medulla secretes epinephrine (Epi) and norepinephrine (NorEpi). 1 Introductory Human Physiology ©copyright Emma Jakoi MINERALOCORTICOIDS The major mineralocorticoid in humans is aldosterone. Aldosterone is NOT under the hypothalamus- pituitary control and does not mediate a negative feedback to this axis. Aldosterone secretion is increased by the vasoconstrictor, angiotensin II, and by elevated plasma K+ concentration. Elevated plasma Na+ inhibits the secretion of aldosterone. Aldosterone, acts in the kidney to promote secretion of K+ into the urine from the blood and the reabsorption of Na+ from the urine into the blood. -

A Case of Bilateral Aldosterone-Producing Adenomas Differentiated by Segmental Adrenal Venous Sampling for Bilateral Adrenal Sparing Surgery
OPEN Journal of Human Hypertension (2016) 30, 379–385 © 2016 Macmillan Publishers Limited All rights reserved 0950-9240/16 www.nature.com/jhh ORIGINAL ARTICLE A case of bilateral aldosterone-producing adenomas differentiated by segmental adrenal venous sampling for bilateral adrenal sparing surgery R Morimoto1, N Satani2, Y Iwakura1, Y Ono1, M Kudo1, M Nezu1, K Omata1,3, Y Tezuka1,3, K Seiji2,HOta2, Y Kawasaki4, S Ishidoya4, Y Nakamura5, Y Arai4, K Takase2, H Sasano5,SIto1 and F Satoh1,3 Primary aldosteronism due to unilateral aldosterone-producing adenoma (APA) is a surgically curable form of hypertension. Bilateral APA can also be surgically curable in theory but few successful cases can be found in the literature. It has been reported that even using successful adrenal venous sampling (AVS) via bilateral adrenal central veins, it is extremely difficult to differentiate bilateral APA from bilateral idiopathic hyperaldosteronism (IHA) harbouring computed tomography (CT)-detectable bilateral adrenocortical nodules. We report a case of bilateral APA diagnosed by segmental AVS (S-AVS) and blood sampling via intra-adrenal first-degree tributary veins to localize the sites of intra-adrenal hormone production. A 36-year-old man with marked long-standing hypertension was referred to us with a clinical diagnosis of bilateral APA. He had typical clinical and laboratory profiles of marked hypertension, hypokalaemia, elevated plasma aldosterone concentration (PAC) of 45.1 ng dl− 1 and aldosterone renin activity ratio of 90.2 (ng dl − 1 per ng ml − 1 h − 1), which was still high after 50 mg-captopril loading. CT revealed bilateral adrenocortical tumours of 10 and 12 mm in diameter on the right and left sides, respectively. -
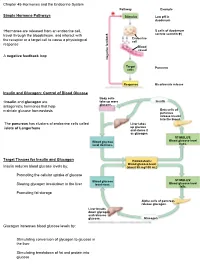
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low Ph in Duodenum
Chapter 45-Hormones and the Endocrine System Pathway Example – Simple Hormone Pathways Stimulus Low pH in duodenum •Hormones are released from an endocrine cell, S cells of duodenum travel through the bloodstream, and interact with secrete secretin ( ) Endocrine the receptor or a target cell to cause a physiological cell response Blood vessel A negative feedback loop Target Pancreas cells Response Bicarbonate release Insulin and Glucagon: Control of Blood Glucose Body cells •Insulin and glucagon are take up more Insulin antagonistic hormones that help glucose. maintain glucose homeostasis Beta cells of pancreas release insulin into the blood. The pancreas has clusters of endocrine cells called Liver takes islets of Langerhans up glucose and stores it as glycogen. STIMULUS: Blood glucose Blood glucose level level declines. rises. Target Tissues for Insulin and Glucagon Homeostasis: Blood glucose level Insulin reduces blood glucose levels by: (about 90 mg/100 mL) Promoting the cellular uptake of glucose Blood glucose STIMULUS: Slowing glycogen breakdown in the liver level rises. Blood glucose level falls. Promoting fat storage Alpha cells of pancreas release glucagon. Liver breaks down glycogen and releases glucose. Glucagon Glucagon increases blood glucose levels by: Stimulating conversion of glycogen to glucose in the liver Stimulating breakdown of fat and protein into glucose Diabetes Mellitus Type I diabetes mellitus (insulin-dependent) is an autoimmune disorder in which the immune system destroys pancreatic beta cells Type II diabetes -

Glossary of Pediatric Endocrine Terms
Glossary of Pediatric Endocrine Terms Adrenal Glands: Triangle-shaped glands located on top of each kidney that are responsible for the regulation of stress response by producing hormones such as cortisol and adrenaline. Adrenal Crisis: A life-threatening condition that results when there is not enough cortisol (stress hormone) in the body. This may present with nausea, vomiting, abdominal pain, severely low blood pressure, and loss of consciousness. Adrenocorticotropin (ACTH): A hormone produced by the anterior pituitary gland that triggers the adrenal gland to produce cortisol, the stress hormone. Anterior Pituitary: The front of the pituitary gland that secretes growth hormone, gonadotropins, thyroid stimulating hormone, prolactin, adrenocorticotropin hormone. Antidiuretic Hormone: A hormone secreted by the posterior pituitary that controls how much water is excreted by the kidneys (water balance). Bone Age Study: An X-ray of the left hand and wrist to assess a child’s skeletal age. It is often used to evaluate disorders of puberty and growth. Congenital Adrenal Hyperplasia: A group of disorders which impair the adrenal steroid synthesis pathway. This causes the body makes too many androgens. Sometimes the body does not make enough cortisol and aldosterone. Constitutional Delay of Growth and Puberty: A normal variant of growth in which children grow at a slower rate and begin puberty later than their peers. They may appear short until they have catch up growth. They usually end up at a normal adult height. A diagnosis of constitutional delay of growth and puberty is often referred to as being a “late bloomer.” Cortisol: The “stress hormone”, which is produced by the adrenal glands. -

Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis
Review Dream Recall/Affect and the Hypothalamic–Pituitary–Adrenal Axis Athanasios Tselebis 1, Emmanouil Zoumakis 2 and Ioannis Ilias 3,* 1 Department of Psychiatry, Sotiria Hospital, 115 27 Athens, Greece; [email protected] 2 First Department of Pediatrics, National and Kapodistrian University of Athens Medical School, Aghia Sophia Children’s Hospital, 115 27 Athens, Greece; [email protected] 3 Department of Endocrinology, Diabetes and Metabolism, Elena Venizelou Hospital, 115 21 Athens, Greece * Correspondence: [email protected]; Tel.: +30-213-205-1389 Abstract: In this concise review, we present an overview of research on dream recall/affect and of the hypothalamic–pituitary–adrenal (HPA) axis, discussing caveats regarding the action of hormones of the HPA axis (mainly cortisol and its free form, cortisol-binding globulin and glucocorticoid receptors). We present results of studies regarding dream recall/affect and the HPA axis under physiological (such as waking) or pathological conditions (such as in Cushing’s syndrome or stressful situations). Finally, we try to integrate the effect of the current COVID-19 situation with dream recall/affect vis-à-vis the HPA axis. Keywords: dreams; cortisol; stress; memory; sleep; HPA axis 1. Introduction—Dream Recall Almost all humans dream (indeed, there may be 0.5% of people who do not do so) [1]. Dreams are a series of images, thoughts and senses and are considered a specific type Citation: Tselebis, A.; Zoumakis, E.; of experience that occurs in our brain during sleep. During the dream, there is limited Ilias, I. Dream Recall/Affect and the control of the dream content, visual images and memory activation. -

Pig Gonads, Adrenal Glands and Brain C
Immunoreactive cytochrome P-45017\g=a\in rat and guinea- pig gonads, adrenal glands and brain C. Le Goascogne1, N. Sanan\l=e'\s1, M. Gou\l=e'\zou1, S. Takemori2, S. Kominami2, E. E. Baulieu1 and P. Robel1 1INSERM U33, Communications Hormonales, and Faculté de Médecine, Université Paris-sud, Lab Hormones F-94275 Bicêtre Cedex France 2 Faculty of Integrated Arts and Sciences, Hiroshima University, Hiroshima 730, Japan Summary. The cytochrome P-45017\g=a\-hydroxylase, 17\ar=r\20lyase (P-45017\g=a\) is the key enzyme responsible for the biosynthesis of androgens in steroidogenic organs. Its cellular localization has been examined with an immunohistochemical technique. In immature rat ovary, P-45017\g=a\was first detected in sparse interstitial cells on postnatal Day 8. The number of immunoreactive interstitial cells increased thereafter and the intensity of P-45017\g=a\staining in these cells was highest at 3 weeks of age. The intensity of staining then started to decline and was very faint at Day 35. From 6 weeks on, the distribution of immunoreactive P-45017\g=a\was of the adult type: it was detected exclusively in the thecal cells of the large antral, preovulatory, follicles. P-45017\g=a\was not detectable during pregnancy except on the day of parturition, when thecal cells were transiently immunoreactive. The staining had vanished 24 h after delivery. Human chorionic gonadotrophin (hCG), injected into immature females on Days 24 to 26, induced P-45017\g=a\prematurely in thecal cells. When injected on Days 12 to 14 of pregnancy, hCG also induced P-45017\g=a\in the thecal cells surrounding the largest follicles, whereas the interstitial and luteal cells were not immunostained.