United States Patent 19 11 Patent Number: 5,360,712 Olm Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

M = Al, Cr, Fe, Ga, In, Sc, Ti, and V) and Fej-J-Mjjfg Mixed Series (M = Ga, Cr, V) *
Systematic Investigation of MF3 Crystalline Compounds (M = Al, Cr, Fe, Ga, In, Sc, Ti, and V) and Fej-j-MjjFg Mixed Series (M = Ga, Cr, V) * H.-R. Blank1, M. Frank1, M. Geiger1, J.-M. Greneche2, M. Ismaier1, M. Kaltenhäuserl, R. Kapp1, W. Kreische1, M. Leblanc3, U. Lossen1, and B. Zapf1 1 Physikalisches Institut der Universität Erlangen-Nürnberg, Erwin-Rommel-Str. 1, 91058 Erlangen, Germany 2 URA CNRS 807, Faculte des Sciences, Universite du Maine, 72017 Le Mans, BP 535, Cedex, France 3 URA CNRS 449, Faculte des Sciences, Universite du Maine, 72017 Le Mans, BP 535, Cedex, France Z. Naturforsch. 49 a, 361-366 (1994); received July 23, 1993 The electric field gradient at the fluorine site of several crystalline trifluorides was measured by means of the time differential perturbed angular distribution method. The hyperfine data (vq and rj) are systematically analyzed by taking into account the structural parameters of the crystals; tney are also compared to the results obtained by point charge calculations. Introduction literature: rhombohedral [2], hexagonal-rhombohe- dral [3] and cubic [4] for 3ScF and rhombohedral or During the last fifteen years, the intrinsic character monoclinic for InF3. These aspects are relevant for the of the fluorine ion, mainly its low polarizability, discussion of experimental data, but the TDPAD hy- evoked much fundamental interest in the physical perfine parameters are not highly sensitive to symme properties of fluorides. In the case of iron based fluo try conditions. rides, the electric field gradient (EFG) at the 57Fe sites After a brief description of the experimental aspects, was determined by Mössbauer spectroscopy [1]. -

Zerohack Zer0pwn Youranonnews Yevgeniy Anikin Yes Men
Zerohack Zer0Pwn YourAnonNews Yevgeniy Anikin Yes Men YamaTough Xtreme x-Leader xenu xen0nymous www.oem.com.mx www.nytimes.com/pages/world/asia/index.html www.informador.com.mx www.futuregov.asia www.cronica.com.mx www.asiapacificsecuritymagazine.com Worm Wolfy Withdrawal* WillyFoReal Wikileaks IRC 88.80.16.13/9999 IRC Channel WikiLeaks WiiSpellWhy whitekidney Wells Fargo weed WallRoad w0rmware Vulnerability Vladislav Khorokhorin Visa Inc. Virus Virgin Islands "Viewpointe Archive Services, LLC" Versability Verizon Venezuela Vegas Vatican City USB US Trust US Bankcorp Uruguay Uran0n unusedcrayon United Kingdom UnicormCr3w unfittoprint unelected.org UndisclosedAnon Ukraine UGNazi ua_musti_1905 U.S. Bankcorp TYLER Turkey trosec113 Trojan Horse Trojan Trivette TriCk Tribalzer0 Transnistria transaction Traitor traffic court Tradecraft Trade Secrets "Total System Services, Inc." Topiary Top Secret Tom Stracener TibitXimer Thumb Drive Thomson Reuters TheWikiBoat thepeoplescause the_infecti0n The Unknowns The UnderTaker The Syrian electronic army The Jokerhack Thailand ThaCosmo th3j35t3r testeux1 TEST Telecomix TehWongZ Teddy Bigglesworth TeaMp0isoN TeamHav0k Team Ghost Shell Team Digi7al tdl4 taxes TARP tango down Tampa Tammy Shapiro Taiwan Tabu T0x1c t0wN T.A.R.P. Syrian Electronic Army syndiv Symantec Corporation Switzerland Swingers Club SWIFT Sweden Swan SwaggSec Swagg Security "SunGard Data Systems, Inc." Stuxnet Stringer Streamroller Stole* Sterlok SteelAnne st0rm SQLi Spyware Spying Spydevilz Spy Camera Sposed Spook Spoofing Splendide -

Name Midterm Review
NAME MIDTERM REVIEW 1. During a laboratory activity, a student combined two solutions. In the laboratory report, the student wrote “A yellow color appeared.” The statement represents the student’s recorded A) conclusion B) observation C) hypothesis D) inference 2. Which of the following statements contained in a student's laboratory report is a conclusion? A) A gas is evolved. B) The gas is insoluble in water. C) The gas is hydrogen. D) The gas burns in air. 3. The diagram below represents a portion of a 100-milliliter graduated cylinder. What is the reading of the meniscus? A) 35.0 mL B) 36.0 mL C) 44.0 mL D) 45.0 mL 4. An aluminum sample has a mass of 80.01 g and a density of 2.70 g/cm3. According to the data, to what number of significant figures should the calculated volume of the aluminum sample be expressed? A) 1 B) 2 C) 3 D) 4 5. A student in a laboratory determined the boiling point of a substance to be 71.8°C. The accepted value for the boiling point of this substance is 70.2°C. What is the percent error of the student's measurement? A) 1.60% B) 2.28% C) 2.23% D) 160.% MIDTERM REVIEW 6. Base your answer to the following question on the information below. A method used by ancient Egyptians to obtain copper metal from copper(I) sulfide ore was heating the ore in the presence of air. Later, copper was mixed with tin to produce a useful alloy called bronze. -
![Determination of the Activation Parameters of Reaction Between [Fe(CN6] and K[Co(HEDTA)NO2]." (2009)](https://docslib.b-cdn.net/cover/8644/determination-of-the-activation-parameters-of-reaction-between-fe-cn6-and-k-co-hedta-no2-2009-998644.webp)
Determination of the Activation Parameters of Reaction Between [Fe(CN6] and K[Co(HEDTA)NO2]." (2009)
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 12-2009 Determination of the Activation Parameters of -4 Reaction Between [Fe(CN6] and K[Co(HEDTA)NO2]. Sammy Eni Eni East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Organic Chemistry Commons Recommended Citation -4 Eni Eni, Sammy, "Determination of the Activation Parameters of Reaction Between [Fe(CN6] and K[Co(HEDTA)NO2]." (2009). Electronic Theses and Dissertations. Paper 1798. https://dc.etsu.edu/etd/1798 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. -4 The Determination of the Activation Parameters of Reaction Between [Fe(CN)6] and K[Co(HEDTA)NO2] ________________________________ A thesis presented to the faculty of the Department of Chemistry East Tennessee State University in Partial Fulfillment of the requirements for the degree Master of Science in Chemistry ________________________________ by Sammy Eni Eni December 2009 ________________________________ Dr. Jeffrey Wardeska, Chair Dr. Ningfeng Zhao Dr. Yu-Lin Jiang -4 Keywords: Activation parameters, [Fe(CN)6] , K[Co(HEDTA)NO2]. ABSTRACT -4 Determination of the Activation Parameters of Reaction Between [Fe(CN)6] and K[Co(HEDTA)NO2] by Sammy Eni Eni -4 The kinetics of the oxidation of [Fe(CN)6] by K[Co(HEDTA)NO2] was studied in order to get the mechanism and the activation parameters of the reaction. -

Reversibility of Ferri-/Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy
Reversibility of Ferri-/Ferrocyanide Redox during Operando Soft X-ray Spectroscopy The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Risch, Marcel, Kelsey A. Stoerzinger, Tom Z. Regier, Derek Peak, Sayed Youssef Sayed, and Yang Shao-Horn. “Reversibility of Ferri-/ Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy.” The Journal of Physical Chemistry C 119, no. 33 (August 20, 2015): 18903–18910. As Published http://dx.doi.org/10.1021/acs.jpcc.5b04609 Publisher American Chemical Society (ACS) Version Author's final manuscript Citable link http://hdl.handle.net/1721.1/109590 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Reversibility of Ferri-Ferrocyanide Redox During Operando Soft X-Ray Spectroscopy ┴ Marcel Risch,†* Kelsey A. Stoerzinger,§ Tom Z. Regier,‡ Derek Peak,º Sayed Youssef Sayed,† Yang Shao-Horn†§||* †Research Laboratory of Electronics, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 §Department of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 ||Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA, USA 02139 ‡Canadian Light Source, Inc., Saskatoon, SK, Canada S7N 2V3 ºDepartment of Soil Sciences, University of Saskatchewan, Saskatoon, SK, Canada S7N 5A8 KEYWORDS. Electrochemistry; In-situ; redox shuttle; iron cyanide; X-ray absorption; radiolysis; radiation damage. ABSTRACT The ferri-ferrocyanide redox couple is ubiquitous in many fields of physical chemistry. We studied its photochemical response to intense synchrotron radiation by in-situ X-Ray absorption spectroscopy. -

Biosynthesis, Characterization and Invitro Antibacterial Activity of Silver Nanoparticles from Seaweeds Against Selected Poultry Pathogens
Indo American Journal of Pharmaceutical Research, 2017 ISSN NO: 2231-6876 BIOSYNTHESIS, CHARACTERIZATION AND INVITRO ANTIBACTERIAL ACTIVITY OF SILVER NANOPARTICLES FROM SEAWEEDS AGAINST SELECTED POULTRY PATHOGENS S. R. Sivakumar*, Bonaventure Mujyambere, G. Dharmaraj *Department of Botany, Bharathidasan University, Trichy-24, Tamilnadu, India. Department of Biotechnology, Nandha Arts and Science College, Erode -638 052, Tamilnadu, India. ARTICLE INFO ABSTRACT Article history In the present study Silver nanoparticles were synthesized from aqueous seaweed extract of Received 12/04/2017 seaweeds such as of green Halimeda macroloba, brown Turbinaria conoides, and red Available online Spyridia filamentosa and characterized by UV-Vis, FTIR, EDX, XRD and SEM. 30/04/2017 Characterization by the above said instrument analysis confirmed the presence,size and stability of silver nanoparticles.After characterization, the silver nanoparticles were tested at Keywords 25µg-ml,50µg-ml,75µg-ml and 100µg-ml concentrations to check the bactericidal activity against Seaweed, selected two poultry bacterial pathogens. We observed that, if the concentration of seaweed Halimeda Macroloba, nanoparticle increases, the zone of inhibition also get increased in all the tested two bacterial Turbinaria Conoides, pathogens against streptomycin as control and result suggested the potential use of seaweed Spyridia Filamentosa Silver synthesized silver nanoparticles against other pathogens. Nanoparticle Synthesis, Characterization, Antibacterial Activity, Selected Poultry Pathogens. Corresponding author Dr. S. R. Sivakumar Assistant Professor, Department of Botany, Bharathidasan Universiy,Trichy-24, Tamilnadu, India. 9345269943/7868088513 [email protected] Please cite this article in press as Dr. S. R. Sivakumar et al. Biosynthesis,Characterization and Invitro Antibacterial Activity of Silver Nanoparticles from Seaweeds Against Selected Poultry Pathogens. -

Investigation of Novel Nanoparticles of Gallium Ferricyanide and Gallium Lawsonate As Potential Anticancer Agents, and Nanoparti
Investigation of Novel Nanoparticles of Gallium Ferricyanide and Gallium Lawsonate as Potential Anticancer Agents, and Nanoparticles of Novel Bismuth Tetrathiotungstate as Promising CT Contrast Agent A Thesis submitted to Kent State University In partial fulfillment of the requirements for the degree of Master of Science Liu Yang August 2014 Thesis written by Liu Yang B.S. Kent State University, 2013 M.S. Kent State University, 2014 Approved by ___________________________________, Advisor, Committee member Dr. Songping Huang ___________________________________, Committee member Dr. Scott Bunge ___________________________________, Committee member Dr. Mietek Jaroniec Accepted by ___________________________________, Chair, Department of Chemistry Dr. Michael Tubergen ___________________________________, Dean, College of Arts and Sciences Dr. James L. Blank ii Table of Contents List of Figures..…………………………………………………………………........vii Acknowledgements ……………………………………………………………….….xi Chapter 1: Summary, Materials and Methods …..……………………………………1 1.1 Materials ………………………………………………………………….3 1.1.1 carboxymethyl reduced polysaccharide (CMRD) preparation….3 1.2 Methods …………………………………………………………………4 1.2.1 Atomic absorption spectroscopy (AA) …………………………4 1.2.2 Acid base treating method ……………………………………...4 1.2.3 Cell viability study ……………………………………………...5 i) MTT assay…………………………………………………..5 ii) Trypan blue assay ………………………………………….6 1.2.4 Dialysis …………………………………………………………6 1.2.5 Elementary analysis …………………………………………….7 1.2.6 Lyophilization …………………………………………………..7 iii 1.2.7 -
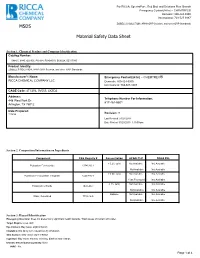
MSDS Material Safety Data Sheet
For RICCA, SpectroPure, Red Bird, and Solutions Plus Brands Emergency Contact(24 hr) -- CHEMTREC® Domestic: 800-424-9300 International: 703-527-3887 ZOBELL'S SOLUTION, APHA ORP Solution, and other ORP Standards MSDS Material Safety Data Sheet Section 1: Chemical Product and Company Identification Catalog Number: 5464.5, 9880, OX-905, PX-918, R5464510, S0932A, SZ117300 Product Identity: ZOBELL'S SOLUTION, APHA ORP Solution, and other ORP Standards Manufacturer's Name: Emergency Contact(24 hr) -- CHEMTREC® RICCA CHEMICAL COMPANY LLC Domestic: 800-424-9300 International: 703-527-3887 CAGE Code: 4TCW6, 0V553, 4XZQ2 Address: Telephone Number For Information: 448 West Fork Dr 817-461-5601 Arlington, TX 76012 Date Prepared: 11/9/98 Revision: 9 Last Revised: 01/24/2014 Date Printed: 03/25/2015 1:19:09 pm Section 2. Composition/Information on Ingredients Component CAS Registry # Concentration ACGIH TLV OSHA PEL < 0.2% (w/v) Not Available Not Available Potassium Ferricyanide 13746-66-2 Not Available Not Available < 0.5% (w/v) Not Available Not Available Potassium Ferrocyanide Trihydrate 14459-95-1 1 (as Fe) mg/m3 Not Available < 1% (w/v) Not Available Not Available Potassium Chloride 7447-40-7 Not Available Not Available Balance Not Available Not Available Water, Deionized 7732-18-5 Not Available Not Available Section 3: Hazard Identification Emergency Overview: Does not present any significant health hazards. Wash areas of contact with water. Target Organs: eyes, skin Eye Contact: May cause slight irritation. Inhalation: Not likely to be hazardous by inhalation. Skin Contact: May cause slight irritation. Ingestion: May cause nausea, vomiting, diarrhea and cramps. -

Chem 110 Fall 2009 Exam I Whelan
Chem 110 Fall 2009 Exam I Whelan SID Last First Question 1 How many significant figures are there in each of the following numbers? 6 Points a. 57.44 c. 3.40x103 b. 0.065 Question 2 a. When 36.456 is added to 74.2, the result should be reported to how many 6 Points decimal places? b. The number 26.71560... rounded to 4 significant figures is: c. Reported to the correct number of significant figures, how many hours are there in exactly 24 days? Question 3 A chemist needs 2.19g of a liquid compound with a density of 0.921 g.cm-3. What volume 5 Points of the compound is required? Show work. cm3 Question 4 Give the correct name for the following polyatomic ions: 8 Points 2- a. Cr2O7 b. CN- - c. ClO4 2- d. CrO4 Question 5 How many protons, neutrons and electrons are there in 18O2- ? 6 Points Protons Neutrons Electrons Question 6 The following questions pertain to the periodic table given at the front of this exam: 8 Points a. The symbol for the noble gas in period 4? b. The symbol for the group VB, period 5 element? c. The symbol for the lightest alkali earth metal is? d. Group VIIA are collective known as: Question 7 1. Name the compound with the formula Al2(SO3)3? 8 Points 2. Name the compound with the formula Cu(NO3)2? 3. What is the formula for magnesium nitride? 4. What is the formula for iron(II) hydroxide? Question 8 A certain element consists of two stable isotopes: 5 Points Exact Mass (amu) Abundance (%) #1 112.9043 4.28 #2 114.9041 95.72 What is the average atomic mass of this element? Give answer to 4 decimal places Show Work amu Question 9 How many MOLES of chlorine are present in 4.05 grams of carbon tetrachloride? 6 Points Show Work moles Question 10 How many GRAMS of I- are present in 2.03 moles of copper(II) iodide? 5 Points Show Work grams Question 11 Balance the following chemical equations using the smallest possible integer coefficients. -

Periodic Classification of the Elements
UNIT 2 Periodic Classification of the Elements Unit Outcomes After completing this unit, you will be able to: ( understand the periodic classification of the elements; ( develop skills in correlating the electron configuration of elements with the periodicity of the elements, and in predicting the trends of periodic properties of elements in the periodic table; ( appreciate the importance of classification in chemistry; and ( demonstrate scientific inquiry skills: observing, inferring, predicting, classifying, comparing and contrasting, making models, communicating, measuring, asking questions, interpreting illustrations, drawing conclusions, applying concepts, and problem solving. MAIN CONTENTS 2.1 Introduction 2.2 The Modern Periodic Table 2.3 Periodic properties in the Periodic Table 2.4 Advantages of periodic classification – Unit Summary – Review Exercises CHEMISTRY GRADE 9 Start-up Activity How can you use a table of repeating events to predict the next events? Table 2.1 shows a familiar table of repeating properties. What is it? Of course, it is a calendar, but it is a calendar with a difference – it is missing some information. You can determine what is missing and fill in the blanks. 1. Look at the calendar again. The calendar has columns of days, Sunday through Saturday. The calendar also has horizontal rows. They are its weeks. 2. Examine the information surrounding each empty spot. Can you tell what information is needed in each empty spot? 3. Fill in the missing information. For example: Conclude and Apply 1. One day in column 3 is marked X, and a day in column 4 is marked Y. What dates belong to these positions? Discuss your answers in your group. -

The Light Triggered Dissolution of Gold Wires Using Potassium Ferrocyanide T Solutions Enables Cumulative Illumination Sensing ⁎ Weida D
Sensors & Actuators: B. Chemical 282 (2019) 52–59 Contents lists available at ScienceDirect Sensors and Actuators B: Chemical journal homepage: www.elsevier.com/locate/snb The light triggered dissolution of gold wires using potassium ferrocyanide T solutions enables cumulative illumination sensing ⁎ Weida D. Chena, Seung-Kyun Kangb, Wendelin J. Starka, John A. Rogersc,d,e, Robert N. Grassa, a Department of Chemistry and Applied Biosciences, ETH Zurich, Vladimir-Prelog-Weg 1, 8093 Zurich, Switzerland b Department of Bio and Brain Engineering, KAIST, 291 Daehak-ro, Yuseong-gu, Daejeon 334141, Republic of Korea c Departments of Materials Science and Engineering, Biomedical Engineering, Neurological Surgery, Chemistry, Mechanical Engineering, Electrical Engineering and Computer Science, Northwestern University, Evanston, IL 60208, USA d Center for Bio-Integrated Electronics, Northwestern University, Evanston, IL 60208, USA e Simpson Querrey Institute for Nano/Biotechnology, Northwestern University, Evanston, IL 60208, USA ARTICLE INFO ABSTRACT Keywords: Electronic systems with on-demand dissolution or destruction capabilities offer unusual opportunities in hard- Photochemistry ware-oriented security devices, advanced military spying and controlled biological treatment. Here, the dis- Cyanide solution chemistry of gold, generally known as inert metal, in potassium ferricyanide and potassium ferrocya- Sensor nide solutions has been investigated upon light exposure. While a pure aqueous solution of potassium Conductors ferricyanide–K3[Fe(CN)6] does not dissolve gold, an aqueous solution of potassium ferrocyanide–K4[Fe(CN)6] Diffusion limitation irradiated with ambient light is able to completely dissolve a gold electrode within several minutes. Photo Devices activation and dissolution kinetics were assessed at different initial pH values, light irradiation intensities and ferrocyanide concentrations. -

Long-Term Ferrocyanide Application Via Deicing Salts Promotes the Establishment of Actinomycetales Assimilating Ferrocyanide-Derived Carbon in Soil
bs_bs_banner Long-term ferrocyanide application via deicing salts promotes the establishment of Actinomycetales assimilating ferrocyanide-derived carbon in soil Silvia Gschwendtner,1 Tim Mansfeldt,2 soils, belonging mostly to Actinomycetales Susanne Kublik,1 Evangelia Touliari,1 (Kineosporia, Mycobacterium, Micromonosporaceae). Franz Buegger3 and Michael Schloter1,* In the soil without pre-exposition, bacteria belonging 1Research Unit Environmental Genomics, Helmholtz to Acidobacteria (Gp3, Gp4, Gp6), Gemmatimonade- Zentrum Munchen,€ German Research Center for tes (Gemmatimonas)andGammaproteobacteria Environmental Health (GmbH), Ingolstadter€ Landstraße 1, (Thermomonas, Xanthomonadaceae)usedferro- Neuherberg 85764, Germany. cyanide as C source but not the present Actinomyc- 2Department Geowissenschaften, Bodengeographie/ etales. This indicated that (i) various bacteria are able Bodenkunde, Universitat€ zu Koln,€ Albertus-Magnus- to assimilate ferrocyanide-derived C and (ii) long-term Platz, Koln€ 50923, Germany. exposition to ferrocyanide applied with deicing salts 3Institute of Biochemical Plant Pathology, Helmholtz leads to Actinomycetales outcompeting other microor- Zentrum Munchen,€ German Research Center for ganisms for the use of ferrocyanide as C source. Environmental Health (GmbH), Ingolstadter€ Landstraße 1, Neuherberg 85764, Germany. Introduction Cyanide is produced by various organisms including Summary bacteria, algae, fungi and higher plants as defence Cyanides are highly toxic and produced by various mechanism or offensive strategy (Møller and Seigler, microorganisms as defence strategy or to increase 1998; Gallagher and Manoil, 2001; Zagrobelny et al., their competitiveness. As degradation is the most 2008) and consequently occurs naturally at low levels in efficient way of detoxification, some microbes devel- the environment. However, it is highly toxic for living oped the capability to use cyanides as carbon and organisms by complexing metalloproteins, e.g.