The Recovery of the Maldivian Reefs After Natural Disasters
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electricity Needs Assessment
Electricity needs Assessment Atoll (after) Island boxes details Remarks Remarks Gen sets Gen Gen set 2 Gen electricity electricity June 2004) June Oil Storage Power House Availability of cable (before) cable Availability of damage details No. of damaged Distribution box distribution boxes No. of Distribution Gen set 1 capacity Gen Gen set 1 capacity Gen set 2 capacity Gen set 3 capacity Gen set 4 capacity Gen set 5 capacity Gen Gen set 2 capacity set 2 capacity Gen set 3 capacity Gen set 4 capacity Gen set 5 capacity Gen Total no. of houses Number of Gen sets Gen of Number electric cable (after) cable electric No. of Panel Boards Number of DamagedNumber Status of the electric the of Status Panel Board damage Degree of Damage to Degree of Damage to Degree of Damaged to Population (Register'd electricity to the island the to electricity island the to electricity Period of availability of Period of availability of HA Fillladhoo 921 141 R Kandholhudhoo 3,664 538 M Naalaafushi 465 77 M Kolhufushi 1,232 168 M Madifushi 204 39 M Muli 764 134 2 56 80 0001Temporary using 32 15 Temporary Full Full N/A Cables of street 24hrs 24hrs Around 20 feet of No High duty equipment cannot be used because 2 the board after using the lights were the wall have generators are working out of 4. reparing. damaged damaged (2000 been collapsed boxes after feet of 44 reparing. cables,1000 feet of 29 cables) Dh Gemendhoo 500 82 Dh Rinbudhoo 710 116 Th Vilufushi 1,882 227 Th Madifushi 1,017 177 L Mundoo 769 98 L Dhabidhoo 856 130 L Kalhaidhoo 680 94 Sh Maroshi 834 166 Sh Komandoo 1,611 306 N Maafaru 991 150 Lh NAIFARU 4,430 730 0 000007N/A 60 - N/A Full Full No No 24hrs 24hrs No No K Guraidhoo 1,450 262 K Huraa 708 156 AA Mathiveri 73 2 48KW 48KW 0002 48KW 48KW 00013 breaker, 2 ploes 27 2 some of the Full Full W/C 1797 Feet 24hrs 18hrs Colappes of the No Power house, building intact, only 80KW generator set of 63A was Distribution south east wall of working. -

Population and Housing Census 2014
MALDIVES POPULATION AND HOUSING CENSUS 2014 National Bureau of Statistics Ministry of Finance and Treasury Male’, Maldives 4 Population & Households: CENSUS 2014 © National Bureau of Statistics, 2015 Maldives - Population and Housing Census 2014 All rights of this work are reserved. No part may be printed or published without prior written permission from the publisher. Short excerpts from the publication may be reproduced for the purpose of research or review provided due acknowledgment is made. Published by: National Bureau of Statistics Ministry of Finance and Treasury Male’ 20379 Republic of Maldives Tel: 334 9 200 / 33 9 473 / 334 9 474 Fax: 332 7 351 e-mail: [email protected] www.statisticsmaldives.gov.mv Cover and Layout design by: Aminath Mushfiqa Ibrahim Cover Photo Credits: UNFPA MALDIVES Printed by: National Bureau of Statistics Male’, Republic of Maldives National Bureau of Statistics 5 FOREWORD The Population and Housing Census of Maldives is the largest national statistical exercise and provide the most comprehensive source of information on population and households. Maldives has been conducting censuses since 1911 with the first modern census conducted in 1977. Censuses were conducted every five years since between 1985 and 2000. The 2005 census was delayed to 2006 due to tsunami of 2004, leaving a gap of 8 years between the last two censuses. The 2014 marks the 29th census conducted in the Maldives. Census provides a benchmark data for all demographic, economic and social statistics in the country to the smallest geographic level. Such information is vital for planning and evidence based decision-making. Census also provides a rich source of data for monitoring national and international development goals and initiatives. -

Base Information Malé, Hulhumalé, Maldives
Base information Malé, Hulhumalé, Maldives We make your most important time of the year to your most beautiful experience. 1 yachts yachts supermarket supermarket Useful information airport Transfer After arrival by plane we pick you up and bring you to the yacht. Please let us know your arrival time. The costs for the transfer are already covered with the comfort package. Address BLUE HORIZON Pte Ltd M.Bolissafaru, 2nd Floor, Orchid Magu, Malé, Maldives GPS: 4.177213, 73.506887 Supermarket Our office can be found in the north of Malé (see map). The yachts are about Near the yachts is a large supermarket 20 minutes away, at Hulhumalé. (Redwave City Square). The two islands are connected by a GPS: 4.211042, 73.542010 bridge and can be reached by taxi or shuttle. Opening hours: daily 09:00 – 18:00 h Contact persons: 20:00 – 22:00 h Base manager: The supermarket can be reached by Mr. Ahmed Zubair Adam taxi, which we gladly organize for 00960 77 88 425 you. The taxi costs about € 5. Office: Mr. Ameer Abbas (00960 794 11 69) Mrs. Lorna (00960 795 11 62) Errors and mistakes reserved. 2 What to do in case of damage? Please contact the base immediately! Exchange insurance policy data (for liability damage) Take pictures of the damage Create a sketch with description of how the accident happened and let Damages can happen even to very experi- it sign from all involved persons enced skippers. Please let us know straight away when damage occurs, so we can Create a record with the port organise everything and so you don’t lose captain valuable holiday time. -

Table 2.3 : POPULATION by SEX and LOCALITY, 1985, 1990, 1995
Table 2.3 : POPULATION BY SEX AND LOCALITY, 1985, 1990, 1995, 2000 , 2006 AND 2014 1985 1990 1995 2000 2006 20144_/ Locality Both Sexes Males Females Both Sexes Males Females Both Sexes Males Females Both Sexes Males Females Both Sexes Males Females Both Sexes Males Females Republic 180,088 93,482 86,606 213,215 109,336 103,879 244,814 124,622 120,192 270,101 137,200 132,901 298,968 151,459 147,509 324,920 158,842 166,078 Male' 45,874 25,897 19,977 55,130 30,150 24,980 62,519 33,506 29,013 74,069 38,559 35,510 103,693 51,992 51,701 129,381 64,443 64,938 Atolls 134,214 67,585 66,629 158,085 79,186 78,899 182,295 91,116 91,179 196,032 98,641 97,391 195,275 99,467 95,808 195,539 94,399 101,140 North Thiladhunmathi (HA) 9,899 4,759 5,140 12,031 5,773 6,258 13,676 6,525 7,151 14,161 6,637 7,524 13,495 6,311 7,184 12,939 5,876 7,063 Thuraakunu 360 185 175 425 230 195 449 220 229 412 190 222 347 150 197 393 181 212 Uligamu 236 127 109 281 143 138 379 214 165 326 156 170 267 119 148 367 170 197 Berinmadhoo 103 52 51 108 45 63 146 84 62 124 55 69 0 0 0 - - - Hathifushi 141 73 68 176 89 87 199 100 99 150 74 76 101 53 48 - - - Mulhadhoo 205 107 98 250 134 116 303 151 152 264 112 152 172 84 88 220 102 118 Hoarafushi 1,650 814 836 1,995 984 1,011 2,098 1,005 1,093 2,221 1,044 1,177 2,204 1,051 1,153 1,726 814 912 Ihavandhoo 1,181 582 599 1,540 762 778 1,860 913 947 2,062 965 1,097 2,447 1,209 1,238 2,461 1,181 1,280 Kelaa 920 440 480 1,094 548 546 1,225 590 635 1,196 583 613 1,200 527 673 1,037 454 583 Vashafaru 365 186 179 410 181 229 477 205 272 -

Common Plants of the Maldives Common Plants Common Plants of the Maldives Is a Starting Point for People Interested in Learning About Trees and Shrubs of the Maldives
series 1 series 1 Common plants of the Maldives Common plants Common Plants of the Maldives is a starting point for people interested in learning about trees and shrubs of the Maldives. It contains of the Maldives descriptions and photographs to help identify local plants as well as information on traditional uses in the Maldives and throughout the world. Whether you’re relaxing in your deck-chair or exploring the island vegetation, you will come to learn that all plants, within every ecosystem are not only beautiful but important for our survival as they provide food, medicine, soil stability, fresh air and water. books in this series are: Common Plants of the Maldives, Common Birds of the Maldives and Life on the Beach, Maldives. series 1 series 1 series 1 Common plants Common birds life on the beach of the Maldives of the Maldives Maldives LIVE&LEARN Environmental Education www.livelearn.org Common plants of the Maldives LIVE&LEARN Environmental Education Haa Alifu Atoll Haa Dhaalu INDIAN OCEAN The Maldives Atoll m There are Shaviyani Atoll approximately 1190 islands in the Maldives with some Noonu Atoll form of vegetation on Raa Atoll them. Lhaviyani Atoll m Approximately 200 are inhabited Baa Atoll islands and 990 are uninhabited. m There are 26 distinct Kaafu Atoll (Malé Atoll) geographical atolls. Alifu Alifu Atoll These are divided MALÉ into 20 administrative regions, with the Alifu Dhaalu Atoll capital Male’ making up a separate Vaavu Atoll administrative unit. Faafu Atoll m The Maldives is 860km long and Meemu Atoll 130km wide. Dhaalu Atoll m More than 99% of the country is water (115,000km2) with Thaa Atoll less than 0.3% land (300km2). -

Coastal Adpatation Survey 2011
Survey of Climate Change Adaptation Measures in Maldives Integration of Climate Change Risks into Resilient Island Planning in the Maldives Project January 2011 Prepared by Dr. Ahmed Shaig Ministry of Housing and Environment and United Nations Development Programme Survey of Climate Change Adaptation Measures in Maldives Integration of Climate Change Risks into Resilient Island Planning in the Maldives Project Draft Final Report Prepared by Dr Ahmed Shaig Prepared for Ministry of Housing and Environment January 2011 Table of Contents 1 INTRODUCTION 1 2 COASTAL ADAPTATION CONCEPTS 2 3 METHODOLOGY 3 3.1 Assessment Framework 3 3.1.1 Identifying potential survey islands 3 3.1.2 Designing Survey Instruments 8 3.1.3 Pre-testing the survey instruments 8 3.1.4 Implementing the survey 9 3.1.5 Analyzing survey results 9 3.1.6 Preparing a draft report and compendium with illustrations of examples of ‘soft’ measures 9 4 ADAPTATION MEASURES – HARD ENGINEERING SOLUTIONS 10 4.1 Introduction 10 4.2 Historical Perspective 10 4.3 Types of Hard Engineering Adaptation Measures 11 4.3.1 Erosion Mitigation Measures 14 4.3.2 Island Access Infrastructure 35 4.3.3 Rainfall Flooding Mitigation Measures 37 4.3.4 Measures to reduce land shortage and coastal flooding 39 4.4 Perception towards hard engineering Solutions 39 4.4.1 Resort Islands 39 4.4.2 Inhabited Islands 40 5 ADAPTATION MEASURES – SOFT ENGINEERING SOLUTIONS 41 5.1 Introduction 41 5.2 Historical Perspective 41 5.3 Types of Soft Engineering Adaptation Measures 42 5.3.1 Beach Replenishment 42 5.3.2 Temporary -

List of MOE Approved Non-Profit Public Schools in the Maldives
List of MOE approved non-profit public schools in the Maldives GS no Zone Atoll Island School Official Email GS78 North HA Kelaa Madhrasathul Sheikh Ibrahim - GS78 [email protected] GS39 North HA Utheem MadhrasathulGaazee Bandaarain Shaheed School Ali - GS39 [email protected] GS87 North HA Thakandhoo Thakurufuanu School - GS87 [email protected] GS85 North HA Filladhoo Madharusathul Sabaah - GS85 [email protected] GS08 North HA Dhidhdhoo Ha. Atoll Education Centre - GS08 [email protected] GS19 North HA Hoarafushi Ha. Atoll school - GS19 [email protected] GS79 North HA Ihavandhoo Ihavandhoo School - GS79 [email protected] GS76 North HA Baarah Baarashu School - GS76 [email protected] GS82 North HA Maarandhoo Maarandhoo School - GS82 [email protected] GS81 North HA Vashafaru Vasahfaru School - GS81 [email protected] GS84 North HA Molhadhoo Molhadhoo School - GS84 [email protected] GS83 North HA Muraidhoo Muraidhoo School - GS83 [email protected] GS86 North HA Thurakunu Thuraakunu School - GS86 [email protected] GS80 North HA Uligam Uligamu School - GS80 [email protected] GS72 North HDH Kulhudhuffushi Afeefudin School - GS72 [email protected] GS53 North HDH Kulhudhuffushi Jalaaludin school - GS53 [email protected] GS02 North HDH Kulhudhuffushi Hdh.Atoll Education Centre - GS02 [email protected] GS20 North HDH Vaikaradhoo Hdh.Atoll School - GS20 [email protected] GS60 North HDH Hanimaadhoo Hanimaadhoo School - GS60 -

For the Installation of an Incinerator at Kulhudhuffushi, Haa Dhaalu Atoll
ENVIRONMENTAL IMPACT ASSESSMENT For the Installation of an Incinerator at Kulhudhuffushi, Haa Dhaalu Atoll By Water Solutions (January 2020) Proposed by: Ministry of Environment Prepared by: Ahmed Jameel (EIA P07/2007), Abdul Aleem (EIA P03/2019) For Water Solutions Pvt. Ltd., Maldives February 2020 EIA for the Installation of an Incinerator at Kulhudhuffushi, Haa Dhaalu Atoll, Maldives 1 Table of contents 1 Table of contents ...................................................................................................... 2 2 List of Figures and Tables ........................................................................................ 6 3 Declaration of the consultants .................................................................................. 8 4 Proponents Commitment and Declaration ............................................................... 9 5 Non-Technical Summary ....................................................................................... 13 6 Introduction ............................................................................................................ 15 6.1 Structure of the EIA ...................................................................................... 15 6.2 Aims and Objectives of the EIA .................................................................... 15 6.3 EIA Implementation ...................................................................................... 15 6.4 Rational for the formulation of alternatives .................................................. 15 6.5 -
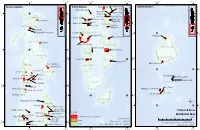
Protected Areas Distribution
73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E Northern Maldives Central Maldives Rasfari beyru Huraa Mangrove Area Southern Maldives Laamu Atoll Rasdhoo Madivaru Girifushi Thila Banana Reef Nassimo Thila 7°0'0"N 7°0'0"N Kuda Haa Lions Head Hans Hass Place; HP Reef Haa Alifu Atoll Mayaa Thila &% Kari beyru Thila Baarah Kulhi Emboodhoo Alifu Alifu Atoll Kanduolhi Orimas Thila 4°0'0"N Kaafu Atoll 4°0'0"N Haa Dhaalu Atoll Fish Head Guraidhoo &% Kanduolhi &% Keylakunu Neykurendhoo Mangrove Hurasdhoo Alifu Dhaalu Atoll 1°0'0"N 1°0'0"N Kudarah Thila Hithaadhoo Rangali Kandu Dhevana Kandu Shaviyani Atoll &% Farukolhu South Ari Atoll MPA Vaavu Atoll Filitheyo Kandu Gaafu Alifu Atoll Vattaru Kandu 6°0'0"N 6°0'0"N Faafu Atoll Noonu Atoll Gaafu Dhaalu Atoll Fushee Kandu Meemu Atoll 3°0'0"N Hakuraa Thila 3°0'0"N Kuredu Express Dhigulaabadhoo Raa Atoll &% Dhaalu Atoll &% Fushivaru Thila 0°0'0" 0°0'0" &% Bathala Region Anemone City &% Lhaviyani Atoll Mendhoo Region Angafaru Thoondi Area Dhandimagu Kilhi &% Maahuruvalhi &% &% &% &% Hanifaru Bandaara Kilhi Thaa Atoll Gnaviyani Atoll Baa Atoll Dhigali Haa &% 5°0'0"N Olhugiri 5°0'0"N Kan'di hera The Wreck of Corbin&% &% Hithadhoo Protected Area Goidhoo Koaru &% Seenu Atoll Mathifaru Huraa British Loyalty 2°0'0"N 2°0'0"N Laamu Atoll Makunudhoo channel &% Kaafu Atoll ¶ Rasfari beyru&% Huraa Mangrove Area 1°0'0"S 1°0'0"S &% Rasdhoo Madivaru &% Girifushi Thila &% Protected Areas &% Nassimo Thila &% Legend Kuda Haa &%Male' CityBanana Reef Kari beyru Thila &% &% Distribution Map Mayaa Thila Lions Head Hans Hass Place Protected Areas 2019 (Total 50 sites) 0 25 50 100 Km &% &% &% Sources: EPA 2019 Alifu Alifu Atoll Emboodhoo Islands Kanduolhi Map version Date: 30/06/2019 &% Orimas Thila Projection: Transverse Mercator (UTM Zone 43 N); 4°0'0"N &% 4°0'0"N Reefs Prepared by: Ministry of Environment, Maldives Fish Head &%Guraidhoo Kanduolhi Horizontal Datum: WGS84; 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E 73°0'0"E 74°0'0"E. -

MINISTRY of TOURISM Approved Opening Dates of Tourist Resorts
MINISTRY OF TOURISM REPUBLIC OF MALDIVES Approved Opening dates of Tourist Resorts, Yacht Marinas, Tourist Hotels, Tourist Vessels, Tourist Guesthouses, Transit Facilities and Foreign Vessels (Updated on 03rd August 2021) TOURIST RESORTS Opening Date No. of No. of No. Facility Name Atoll Island Approved by Beds Rooms MOT Four Seasons Private Island 1 Baa Voavah 26 11 In operation Maldives at Voavah Four Seasons Resort Maldives at 2 Baa Landaa Giraavaru 244 116 In operation Landaa Giraavaru Alifu 3 Lily Beach Resort Huvahendhoo 250 125 In operation Dhaalu 4 Lux North Male' Atoll Kaafu Olhahali 182 91 In operation 5 Oblu By Atmosphere at Helengeli Kaafu Helengeli 236 116 In operation 6 Soneva Fushi Resort Baa Kunfunadhoo 286 143 In operation 7 Varu Island Resort Kaafu Madivaru 244 122 In operation Angsana Resort & Spa Maldives – 8 Dhaalu Velavaru 238 119 In operation Velavaru 9 Velaa Private Island Maldives Noonu Fushivelaavaru 134 67 In operation 10 Cocoon Maldives Lhaviyani Ookolhu Finolhu 302 151 15-Jul-20 Four Seasons Resort Maldives at 11 Kaafu Kuda Huraa 220 110 15-Jul-20 Kuda Huraa 12 Furaveri Island Resort & Spa Raa Furaveri 340 170 15-Jul-20 13 Grand Park Kodhipparu Maldives Kaafu Kodhipparu 250 125 15-Jul-20 14 Hard Rock Hotel Maldives Kaafu Akasdhoo 396 198 15-Jul-20 15 Kudafushi Resort & Spa Raa Kudafushi 214 107 15-Jul-20 Oblu Select by Atmosphere at 16 Kaafu Akirifushi 288 114 15-Jul-20 Sangeli 17 Sun Siyam Olhuveli Maldives Kaafu Olhuveli 654 327 15-Jul-20 18 Ozen By Atmosphere At Maadhoo Kaafu Maadhoo 214 107 15-Jul-20 19 -

High Tide Situation in Some Islands
JOINT RAPID ASSESSMENT REPORT ON SEA SWELL AFFECTED AREAS CONDUCTED BY GOVERNMENTGOVERNMENT of the MALDIVESMALDIVES -– UNUN -- IFRCIFRC JOINT RAPID ASSESSME NT REPORT TIDAL SURGE19 May AFFECTED 2007 AREAS 19 May 2007 National Disaster Management Centre Male’ Republic of Maldives 1 FIG-01 MAP OF MALDIVES WITH THE AFFECTED ISLAND HAA ALIFU ATOLL HAA DHAALU ATOLL SH AVI YA N I ATOLL NOONU ATOLL RAA ATOLL LHAVIYANI ATOLL BAA ATOLL KAAFU ATOLL ALIFU ALIFU ATOLL SOUTH MALE’ ATOLL ALIFU DHAALU ATOLL VAAVU ATOLL FAAFU ATOLL MEEMU ATOLL DHAALU ATOLL THAA ATOLL LAAMU ATOLL Madaveli Hoadedhdhoo Nadallaa Rathafandhoo GAAFU ALIIFU ATOLL Fiyoari Fares-Maathoda GAAFU DHAALU ATOLL GAAFU DHAALU ATOLL GN AVI YA N I ATOLL SEENU ATOLL 2 The full report prepared by the Department of Meteorology is available on request from the Department of Meteorology, email: [email protected], or the National Disaster Management Centre. [email protected]. 3 Executive Summary On 15th May, and for four subsequent days, powerful swells hit many islands throughout the Maldives. According to the Department of Meteorology, the swells were generated by waves between 10 - 15 feet from a dissipated polar storm 3,500 miles south west of Maldives (near 50S, off the coast of South Africa). After generation, the waves traveled northeast for a few thousands kilometres and for a few days (the longest wavelengths travel the fastest) across the Indian Ocean. Following appeals from the southern island and atoll authorities in the Maldives archipelago, a Joint Rapid Assessment mission traveled to the most severely affected islands in Gaafu Dhaalu Atoll on 19th May. -

Effects of Climate Change on Children in the Maldives Justyna Orłowska
Effects of Climate Change on Children in the Maldives Justyna Orłowska CONTENTS: I List of Photos II List of Tables III List of Diagrams 1. Introduction 1.1. Climate change in the context of Maldives’ vulnerability 1.2. Children in Maldives 1.3. Scope of potential effects of climate change to children 1.4. Similar case studies 2. Research Methods 2.1. Aim of the study and research questions 2.2. Research settings 2.3. Sampling and organization 2.4. Research tools 2.5. Challenges and limitations 3. Results of the study 3.1. Awareness of climate change of parents and children 3.2. Effects of climate change to children in Maldives 3.2.1. Health-related effects of climate change 3.2.2. Social and psychological effects of climate change 3.3. Awareness and evaluation of the existing projects 4. Recommendations and conclusions 4.1. Conclusions of the study 4.2. Recommendation for actions 4.3. Recommendation for further studies 5. Bibliography I List of Photos Photo 1: Waste burning producing toxic fumes; Credit: Justyna Orłowska Photo 2: Boy helping his friend to load water buckets; Credit: Aminath Shooza Photo 3: Flooded roads; Credit: Aminath Shooza Photo 4: Harbour project in S. Hithadhoo; Credit: Aminath Shooza Photo 5: Coffee shop named after the 2004 tsunami in H.Dh. Hanimaadhoo; Credit: Aminath Shooza II List of Tables Table 1. Summary of the collected interviews III List of Diagrams Diagram 1: Direct and indirect impacts of climate change on children Diagram 2. Map of the field work Diagram 3: Climate change effects as seen by children and parents Diagram 4: Warning system in Maldives 1.