Case No COMP/M.1403 - ASTRA / ZENECA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biotechnology and the Economics of Discovery in the Pharmaceutical Industry
BIOTECHNOLOGY AND THE ECONOMICS OF DISCOVERY IN THE PHARMACEUTICAL INDUSTRY HELEN SIMPSON Office of Health Economics 12 Whitehall London SWlA 2DY ©October 1998. Office of Health Economics. Price £7.50 ISBN 1 899040 60 9 Printed by BSC Print Ltd, London. About the Author Helen Simpson is currently a researc~ economist at the Institute for Fiscal Studies and was formerly an economist at the Department of Trade and Industry. However, the opinions expressed here are her own and do not necessarily reflect the views of the IFS or of DTI officials or ministers. Acknowledgements This paper has been developed from my MPhil Economics thesis Scientist Entrepreneurs and the Finance of Biotech Companies. I would like to thank Margaret Meyer, Paul David and Gervas Huxley for their valuable suggestions. I am particularly grateful to Hannah Kettler and Jon Sussex for their advice and editorial inputs to the paper. My thanks also go to Adrian Towse and members of the OHE Editorial Board for their comments, and to the following individuals who gave me their insights into the pharmaceutical industry: Dr Trevor Jones, Director General, ABPI; Dr Janet Dewdney, Chairman, Adprotech; Dr Clive Halliday, Head of Global External Scientific Affairs, Glaxo Wellcome; Mr Alan Galloway, Head of Research Administration, Dr Nick Scott-Ram, Director of Corporate Affairs, and Dr Philip Huxley, all of British Biotech; Christine Soden, Finance Director, Chiroscience; Ian Smith, Lehman Brothers Pharmaceutical Research; and Paul Murray, 31. The Office of Health Economics Terms of Reference The Office of Health Economics (OHE) was founded in 1962. Its terms of reference are to: • commission and undertake research on the economics of health and health care; • collect and analyse health and health care data from the UK and other countries; • disseminate the results of this work and stimulate discussion of them and their policy implications. -

Programme 10.00 Registration 10.20 Welcome from the Host
Leadership Seminar: Respiratory and Inflammatory Diseases 22 September 2016 Penningtons Manches, 125 Wood Street, London EC2V 7AW Sponsored by Hosted by Programme 10.00 Registration 10.20 Welcome from the Host 10.30 Introduction Adrian Dawkes, PharmaVentures 10.45 Keynote presentations and discussion on unmet clinical needs including: 10.45 Symptoms vs Disease Modification Approach Lars Larson, TranScrip 11.05 Prospects for the prevention and treatment of respiratory virus-induced exacerbations in asthma and COPD Garth Rapeport, Pulmocide 11.25 Eosinophil depletion with benralizumab (anti-IL-5R) for the management of Severe Asthma and Chronic Obstructive Pulmonary Disease (COPD) Suzanne Cohen, MedImmune 11.45 Breath Biopsy - Breath Volatile Organic Compounds (VOCs) as Markers for Respiratory Disease Billy Boyle, Owlstone Medical 12.05 Panel discussion and Q&A 12.30 Lunch and networking 14.00 Data Protection and Liability in Respiratory Connected Devices Oliver Bett, Penningtons Manches 14.20 Adaptive Design in Respiratory Clinical Trials – A Sponsor’s Business Case Alethea Wieland, Scope International 14.40 Development of Immunoassays and Point-of-Care Tests for the Measurement of Active Protease Biomarkers of Chronic Respiratory Disease David Ribeiro, ProAxsis 15.00 Aiming for the Lungs - Formulation Strategies for Delivery of Inhaled Biologics Charlotte Yates, Vectura 15.30 Tea, coffee and networking 16.00 Innovative therapeutic options 16.00 The Development of an IL-17BR therapeutic antibody for the treatment of Asthma and IPF David Matthews, MRC Technology 16.20 Mycobacterium Tuberculosis Derived Peptide as a Disease Modifying Therapy for Asthma Nicky Cooper, Peptinnovate 16.40 Closing Remarks and Drinks Reception 18.00 Event closes Speaker Profiles Oliver Bett Associate, Penningtons Manches Oliver is an associate in our IP, IT and commercial team of Penningtons Manches, based in the London office. -

Introducing the IMED Biotech Unit What Science Can Do Introduction What Science Can Do
Introducing the IMED Biotech Unit What science can do Introduction What science can do At AstraZeneca, our purpose is to push the Our IMED Biotech Unit applies its research and Our approach to R&D development capabilities and technologies to The IMED Biotech Unit plays a critical boundaries of science to deliver life-changing accelerate the progress of our pipeline. Through role in driving AstraZeneca’s success. Working together with MedImmune, medicines. We achieve this by placing science great collaboration across our three science units, our global biologics arm and Global we are confident that we can deliver the next wave Medicines Development (GMD), our at the centre of everything we do. late-stage development organisation, of innovative medicines to transform the lives of we are ensuring we deliver an innovative patients around the world. and sustainable pipeline. Pancreatic beta cells at different Eosinophil prior to Minute pieces of circulating tumour DNA stages of regeneration apoptosis (ctDNA) in the bloodstream IMED Biotech Unit MedImmune Global Medicines Development Focuses on driving scientific advances Focuses on biologics research and Focuses on late-stage development in small molecules, oligonucleotides and development in therapeutic proteins, of our innovative pipeline, transforming other emerging platforms to push the monoclonal antibodies and other next- exciting science into valued new boundaries of medical science. generation molecules to attack a range medicines and ensuring patients of diseases. around the world can access them. It’s science that compels us to push the boundaries of what is possible. We trust in the potential of ideas and pursue them, alone and with others, until we have transformed the treatment of disease. -

United States Securities and Exchange Commission Form 10-K Shire Pharmaceuticals Group
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ፤ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 អ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PHARMACEUTICALS GROUP PLC (Exact name of registrant as specified in its charter) England and Wales (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) N.A. East Anton, Andover, Hampshire SP10 5RG England (Address of principal executive offices) (Zip Code) 44 1264 333455 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depository Shares, each representing Nasdaq National Market 3 Ordinary Shares, 5 pence nominal value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ፤ No អ Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference to Part III of this Form 10-K or any amendment to this Form 10-K. -

Appendix One the CELLTECH CASE STUDY
City Research Online City, University of London Institutional Repository Citation: Mc Namara, P. (2000). Managing the tension between knowledge exploration and exploitation: the case of UK biotechnology. (Unpublished Doctoral thesis, City University London) This is the accepted version of the paper. This version of the publication may differ from the final published version. Permanent repository link: https://openaccess.city.ac.uk/id/eprint/7870/ Link to published version: Copyright: City Research Online aims to make research outputs of City, University of London available to a wider audience. Copyright and Moral Rights remain with the author(s) and/or copyright holders. URLs from City Research Online may be freely distributed and linked to. Reuse: Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. City Research Online: http://openaccess.city.ac.uk/ [email protected] Managing the Tension Between Knowledge Exploration and Exploitation: The Case of UK Biotechnology By Peter Mc Namara Presented in fulfilment of the requirements of the: Degree of Doctor of Philosophy Strategy and International Business City University Business School Department of Strategy and Marketing March 2000 TABLE OF CONTENTS ACKNOWLEDGEMENTS .......................................................................................................................... -

SHIRE PLC (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K [X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 2009 [ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PLC (Exact name of registrant as specified in its charter) Jersey (Channel Islands) 98-0601486 (State or other jurisdiction of incorporation or (I.R.S. Employer Identification No.) organization) 5 Riverwalk, Citywest Business Campus, Dublin +353 1 429 7700 24, Republic of Ireland (Address of principal executive offices and zip code) (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depositary Shares, each representing three NASDAQ Global Select Market Ordinary Shares 5 pence par value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) 1 Indicate by check mark whether the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act Yes [X] No [ ] Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act Yes [ ] No [X] Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Astrazeneca Plc
6/15/2020 AstraZeneca - Wikipedia AstraZeneca AstraZeneca plc[3] is a British-Swedish multinational pharmaceutical and biopharmaceutical company with its global AstraZeneca plc headquarters in Cambridge, England.[4] Its R&D is concentrated in Cambridge, Gaithersburg, Maryland, and Mölndal in Sweden.[5] AstraZeneca has a portfolio of products for major disease areas including cancer, cardiovascular, gastrointestinal, infection, Type Public limited neuroscience, respiratory and inflammation.[6] company Traded as LSE: AZN (https:// The company was founded in 1999 through the merger of the www.londonstocke [7][8] Swedish Astra AB and the British Zeneca Group (itself formed xchange.com/exch by the demerger of the pharmaceutical operations of Imperial ange/searchengin Chemical Industries in 1993). Since the merger it has been among e/search.html?lang the world's largest pharmaceutical companies and has made =en&x=0&y=0&q= numerous corporate acquisitions, including Cambridge Antibody AZN) Technology (in 2006), MedImmune (in 2007), Spirogen (in 2013) NYSE: AZN (http and Definiens (by MedImmune in 2014). s://www.nyse.com/ quote/XNYS:AZN) AstraZeneca has a primary listing on the London Stock Exchange Nasdaq and is a constituent of the FTSE 100 Index. It has secondary listings Stockholm: AZN (ht on the New York Stock Exchange and the OMX exchange. tp://www.nasdaqom xnordic.com/aktier/ microsite?language Contents Id=1&Instrument=S SE3524) History FTSE 100 2000–06 Component 2007–12: The patent cliff and subsequent acquisitions ISIN GB0009895292 2013 -

Pharmaceuticals in the Environment
Pharmaceuticals in the Environment As a responsible healthcare company, we are committed to the health and safety of our society and planet. We make it a priority to effectively manage the risks associated with pharmaceuticals in the environment (PIE). 1 How do Pharmaceutical Products get into the Environment? Pharmaceuticals enter the environmental mainly as AstraZeneca supports actions based on scientific a result of patient use, where they can pass through evidence to address the challenges presented by PIE. our bodies and into waterways. Drug manufacture and These include: the improper disposal of unused medicines also add • Assessing the environmental risk of our medicines to the trace levels of pharmaceuticals in rivers, lakes, to support drug marketing authorisations soils, and, sometimes, drinking water. AstraZeneca recognises that, even in such low concentrations, • Actively managing the environmental risks resulting the risks associated with Pharmaceuticals in the from our manufacturing Environment (PIE) should be determined, minimised • Supporting industry and government efforts to and managed. improve medicine disposal programs and education • Co-sponsoring research to fill scientific gaps 88% to understand and mitigate the risks of PIE. as a result of patient use To learn more about the risks associated with PIE and what we are doing to manage them please click here. 2% attributed to waste Pharmaceuticals from production in the environment 10% from unused medicines that people don’t dispose of properly 2 Societal Concerns about PIE Trace amounts of pharmaceuticals have been detected in the environment for more than 20 years. As environmental monitoring expands and the methods for measuring pharmaceuticals in the environment become more sophisticated and detection limits get better, the geographical scope and number of pharmaceuticals measured in the environment will grow. -

Monoclonals 5/10/06 11:51 Page 17
Monoclonals 5/10/06 11:51 Page 17 Therapeutics MONOCLONALS the billion dollar molecules of the future In 1975, two British scientists thought of creating a mouse antibody that could be replicated or ‘cloned’ to produce identical copies. Identical copies with identical modes of action had the potential to be a drug.They had launched what was to be one of biotechnology’s best ideas. If the pair could mimic the immune system’s ‘seek and destroy’ capability by pre-designing antibodies for all manner of disease targets, it should be possible to block or activate cellular activity to order. It was an elegant concept opening up a raft of therapeutic possibilities, particularly in the area of cancer. Here it might be possible to attach a chemotherapy drug to an antibody.The antibody’s specificity for a particular disease target would guide the chemo drug only to the cancerous cells and not the healthy ones.The concept, therefore, was excellent, but the use of mice monoclonals had drawbacks.The human body did not like antibodies from mice anymore than it liked the flu virus, so the antibodies themselves triggered an immune response and were destroyed.This ‘immunogenicity’ issue was to occupy researchers for another 20 or so years before it became possible to craft a monoclonal antibody (mAb) that would be acceptable to the human immune system. fter rather a long period in the desert, untreatable diseases. A further six lead drugs are in By Dr Martin Wiles monoclonal antibody drugs are once the final stages of clinical trials and approximately and Patrik A again generating a wave of excitement. -
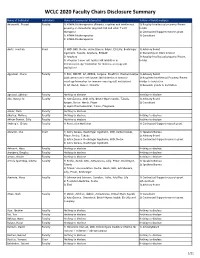
WCLC 2020 Faculty Chairs Disclosure Summary
WCLC 2020 Faculty Chairs Disclosure Summary Name of Individual Individual's Name of Commercial Interest(s) Nature of Relationship(s) Adusumilli, Prasad FacultyRole(s) in Activity 1) ATARA Biotherapeutics (Patents, royalties and intellectual 1) Royalty/Intellectual property/Patent property on mesothelin-targeted CAR and other T-cell holder therapies) 2) Contracted/Support research grant 2) ATARA Biotherapeutics 3) Consultant 3) ATARA Biotherapeutics Aerts, Joachim Chair 1) MSD, BMS, Roche, Astra-Zeneca, BAyer, Eli-Lilly, Boehringer 1) Advisory Board Ingelheim, Takeda, Amphera, BIOCAD 2) Ownership or Stock interest 2) Amphera 3) Royalty/Intellectual property/Patent 3) allogenic tumor cell lysate/JAK inhibition in holder immunooncology/ biomarker for immuno-oncology (all institution) Aggarwal, Charu Faculty 1) BMS, ROCHE, AZ, MERCK, Celgene, BluePrint, Diachaii Sankyo 1) Advisory Board 2)Allogenic tumor cell lysate/JAK inhibition in immuno- 2) Royalties/Intellectual Property/Patent oncology/biomarker for immuno-oncology (all institution) Holder to institution 3) AZ, Merck, Xencor, Novartis 3) Research grants to institution Agrawal, Abhinav Faculty Nothing to disclose Nothing to disclose Ahn, Myung-Ju Faculty 1) AstraZeneca, MSD, Lilly, Bristol-Myers Squibb, Takeda, 1) Advisory Board Amgen, Roche, Merck, Pfizer 2) Consultant 2) Alpha Pharmaceutical, Yuhan, Progenere Aisner, Dara Faculty Nothing to disclose Akerley, Wallace Faculty Nothing to disclose Nothing to disclose Akhtar-Danesh, Gilly Faculty Nothing to disclose Nothing to disclose -

Consolidation Revisited Is the Founder of Managing Resources, a Date Received *In Revised Form) 8Th October, 2001 Consultancy Focused on the Life Science Industries
Alan Williams Consolidation revisited is the founder of Managing Resources, a Date received *in revised form) 8th October, 2001 consultancy focused on the life science industries. The Alan Williams company undertakes strategic assignments and also assists public and private sector clients with regulatory affairs, investor Abstract In 1998, the author produced two papers that argued that consolidation was relations and human a necessary activity for biotechnology companies to pay greater attention to. Three resources development. years later a further consideration of the subject seems worthwhile. Examples of Alan has also been an investment manager in successful consolidators are reviewed. a venture capital company, and held Keywords: consolidation, mergers, acquisitions marketing and licensing positions in Fisons. Introduction drink, vaccines and animal health but there is now greater focus on the highest In the early/mid-1970s, the pharmaceutical pro®tability sector, human pharmaceuticals. industry consisted of more than 100 At the time of writing, GSK is rumoured to research-based businesses of some be a possible purchaser of Bayer's signi®cant size, although none of them pharmaceutical business, the future of could convincingly claim a global presence. which has been questioned after the Typically, a leading company had a strong withdrawal of cerivastatin. position in its local market "examples Aventis, the eighth largest pharmaceutical included Merck and P®zer in the USA, company by sales in 2000,1 includes the Bayer in Germany, Beecham in the UK, operations of 1970s companies such as Roussel-Uclaf in France and Fujisawa in Rhone-Poulenc, Rorer, Fisons, Hoechst, Japan) and possibly some other Marion, Richardson Merrell, and some neighbouring countries "US companies in smaller companies. -

Pharma &Biotech
PHARMA & BIOTECH 1/ 2016 I N Markets & Companies Innovation Manufacturing & Supply Chain Top 10 Brands in the Pharmaceuti- How the Pharma Industry Can Developing New Synthetic Routes, cal Industry, Pharma M&A Update, Bridge the Innovation Gap, Trends High-Potency Manufacturing, Drug Market Reports, Expert Opinions, and Success Factors in Chemical Shortage Prevention, Specialized Company News and Pharmaceutical Research Pharma Logistics Azelis Pharma 2016 advert 240x330_PRINT:Layout 1 02/09/2016 11:38 Page 1 Azelis Pharma – We’re here to add value to your business where it counts In a dynamic pharmaceutical market driven by constant development, changing regulations and evolving healthcare demands, you need a fully compliant partner with proven technical expertise, market knowledge and the capability to keep you more than one step ahead. Azelis serves the human, veterinary health and medical sectors across EMEA, Americas and Asia Pacifi c and represents many of the industry’s top API and excipient manufacturers. Our unrivalled product portfolio is supported by a full package of certifi cations and licenses. APIs – broad therapeutic range includes: cardiovascular, CNS, dermatology, diabetes, infections, nutrition, ophthalmology & pain Excipients – covering all functions: bases, binders, coatings, colours, diluents, fi llers, glidants, lubricants, sorbents, preservatives & sweeteners We work closely with customers to understand their formulation needs, identify new opportunities from patent and therapeutic targeting and proactively add value from product development through to manufacture. Contact us to fi nd out how Azelis can help with quality ingredient sourcing and supply. www.azelis.com · [email protected] Creating value, growing together E DITORIAL Diagnosis: Innovation Insufficiency – Treatment: Combination Therapy Since the beginning of the 21st century, pharma M&A activity has exploded.