Forest Insect & Disease Leaflet
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lepidoptera of North America 5
Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Lepidoptera of North America 5. Contributions to the Knowledge of Southern West Virginia Lepidoptera by Valerio Albu, 1411 E. Sweetbriar Drive Fresno, CA 93720 and Eric Metzler, 1241 Kildale Square North Columbus, OH 43229 April 30, 2004 Contributions of the C.P. Gillette Museum of Arthropod Diversity Colorado State University Cover illustration: Blueberry Sphinx (Paonias astylus (Drury)], an eastern endemic. Photo by Valeriu Albu. ISBN 1084-8819 This publication and others in the series may be ordered from the C.P. Gillette Museum of Arthropod Diversity, Department of Bioagricultural Sciences and Pest Management Colorado State University, Fort Collins, CO 80523 Abstract A list of 1531 species ofLepidoptera is presented, collected over 15 years (1988 to 2002), in eleven southern West Virginia counties. A variety of collecting methods was used, including netting, light attracting, light trapping and pheromone trapping. The specimens were identified by the currently available pictorial sources and determination keys. Many were also sent to specialists for confirmation or identification. The majority of the data was from Kanawha County, reflecting the area of more intensive sampling effort by the senior author. This imbalance of data between Kanawha County and other counties should even out with further sampling of the area. Key Words: Appalachian Mountains, -

Hymenoptera: Symphyta: Pamphiliidae, Siricidae, Cephidae) from the Okanagan Highlands, Western North America S
1 New early Eocene Siricomorpha (Hymenoptera: Symphyta: Pamphiliidae, Siricidae, Cephidae) from the Okanagan Highlands, western North America S. Bruce Archibald,1 Alexandr P. Rasnitsyn Abstract—We describe three new genera and four new species (three named) of siricomorph sawflies (Hymenoptera: Symphyta) from the Ypresian (early Eocene) Okanagan Highlands: Pamphiliidae, Ulteramus republicensis new genus, new species from Republic, Washington, United States of America; Siricidae, Ypresiosirex orthosemos new genus, new species from McAbee, British Columbia, Canada; and Cephidae, Cuspilongus cachecreekensis new genus, new species from McAbee and another cephid treated as Cephinae species A from Horsefly River, British Columbia, Canada. These are the only currently established occurrences of any siricomorph family in the Ypresian. We treat the undescribed new siricoid from the Cretaceous Crato Formation of Brazil as belonging to the Pseudosiricidae, not Siricidae, and agree with various authors that the Ypresian Megapterites mirabilis Cockerell is an ant (Hymenoptera: Formicidae). The Miocene species Cephites oeningensis Heer and C. fragilis Heer, assigned to the Cephidae over a century and a half ago, are also ants. Many of the host plants that siricomporphs feed upon today first appeared in the Eocene, a number of these in the Okanagan Highlands in particular. The Okanagan Highlands sites where these wasps were found also had upper microthermal mean annual temperatures as are overwhelmingly preferred by most modern siricomorphs, but were uncommon -

(Hymenoptera) from the Middle Jurassic of Inner Mongolia, China
European Journal of Taxonomy 733: 146–159 ISSN 2118-9773 https://doi.org/10.5852/ejt.2021.733.1229 www.europeanjournaloftaxonomy.eu 2021 · Zheng Y. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:043C9407-7E8A-4E8F-9441-6FC43E5A596E New fossil records of Xyelidae (Hymenoptera) from the Middle Jurassic of Inner Mongolia, China Yan ZHENG 1,*, Haiyan HU 2, Dong CHEN 3, Jun CHEN 4, Haichun ZHANG 5 & Alexandr P. RASNITSYN 6,* 1,4 Institute of Geology and Paleontology, Linyi University, Shuangling Rd., Linyi 276000, China. 1,4,5 State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology, East Beijing Road, Nanjing 210008, China. 2 School of Agronomy and Environment, Weifang University of Science and Techonoly, Jinguang Road, Shouguang, 262700, China. 3 School of Environmental and Municipal Engineering, Qingdao University of Technology, Qingdao 266033, China. 6 Palaeontological Institute, Russian Academy of Sciences, Moscow, 117647, Russia. 6 Natural History Museum, London SW7 5BD, UK. * Corresponding authors: [email protected], [email protected] 2 Email: [email protected] 3 Email: [email protected] 4 Email: [email protected] 5 Email: [email protected] 1 urn:lsid:zoobank.org:author:28EB8D72-5909-4435-B0F2-0A48A5174CF9 2 urn:lsid:zoobank.org:author:91B2FB61-31A9-449B-A949-7AE9EFD69F56 3 urn:lsid:zoobank.org:author:51D01636-EB69-4100-B5F6-329235EB5C52 4 urn:lsid:zoobank.org:author:8BAB244F-8248-45C6-B31E-6B9F48962055 5 urn:lsid:zoobank.org:author:18A0B9F9-537A-46EF-B745-3942F6A5AB58 6 urn:lsid:zoobank.org:author:E7277CAB-3892-49D4-8A5D-647B4A342C13 Abstract. -

Pine Sawflies, Neodiprion Spp. (Insecta: Hymenoptera: Diprionidae)1 Wayne N
EENY317 Pine Sawflies, Neodiprion spp. (Insecta: Hymenoptera: Diprionidae)1 Wayne N. Dixon2 Introduction Pine sawfly larvae, Neodiprion spp., are the most common defoliating insects of pine trees, Pinus spp., in Florida. Sawfly infestations can cause growth loss and mortality, especially when followed by secondary attack by bark and wood-boring beetles (Coleoptera: Buprestidae, Cerambycidae, Scolytidae). Trees of all ages are susceptible to sawfly defoliation (Barnard and Dixon 1983; Coppel and Benjamin 1965). Distribution Neodiprion spp. are indigenous to Florida. Host tree specificity and location will bear on sawfly distribution statewide. Description Six species are covered here so there is some variation in appearance. However, an adult female has a length of 8 to 10 mm, with narrow antennae on the head and a stout and Figure 1. Larvae of the blackheaded pine sawfly, Neodiprion excitans thick-waisted body. This is unlike most Hymenopteran Rohwer, on Pinus sp. Credits: Arnold T. Drooz, USDA Forest Service; www.forestryimages.org insects which have the thinner, wasp-like waist. The background color varies from light to dark brown, with Adult yellow-red-white markings common. The two pairs of The adult male has a length of 5 to 7 mm. The male has wings are clear to light brown with prominent veins. broad, feathery antennae on the head with a slender, thick- waisted body. It generally has brown to black color wings, similar to the female. 1. This document is EENY317 (originally published as DPI Entomology Circular No. 258), one of a series of the Department of Entomology and Nematology, UF/IFAS Extension. Original publication date January 2004. -

Butterflies and Moths of Dorchester County, Maryland, United States
Heliothis ononis Flax Bollworm Moth Coptotriche aenea Blackberry Leafminer Argyresthia canadensis Apyrrothrix araxes Dull Firetip Phocides pigmalion Mangrove Skipper Phocides belus Belus Skipper Phocides palemon Guava Skipper Phocides urania Urania skipper Proteides mercurius Mercurial Skipper Epargyreus zestos Zestos Skipper Epargyreus clarus Silver-spotted Skipper Epargyreus spanna Hispaniolan Silverdrop Epargyreus exadeus Broken Silverdrop Polygonus leo Hammock Skipper Polygonus savigny Manuel's Skipper Chioides albofasciatus White-striped Longtail Chioides zilpa Zilpa Longtail Chioides ixion Hispaniolan Longtail Aguna asander Gold-spotted Aguna Aguna claxon Emerald Aguna Aguna metophis Tailed Aguna Typhedanus undulatus Mottled Longtail Typhedanus ampyx Gold-tufted Skipper Polythrix octomaculata Eight-spotted Longtail Polythrix mexicanus Mexican Longtail Polythrix asine Asine Longtail Polythrix caunus (Herrich-Schäffer, 1869) Zestusa dorus Short-tailed Skipper Codatractus carlos Carlos' Mottled-Skipper Codatractus alcaeus White-crescent Longtail Codatractus yucatanus Yucatan Mottled-Skipper Codatractus arizonensis Arizona Skipper Codatractus valeriana Valeriana Skipper Urbanus proteus Long-tailed Skipper Urbanus viterboana Bluish Longtail Urbanus belli Double-striped Longtail Urbanus pronus Pronus Longtail Urbanus esmeraldus Esmeralda Longtail Urbanus evona Turquoise Longtail Urbanus dorantes Dorantes Longtail Urbanus teleus Teleus Longtail Urbanus tanna Tanna Longtail Urbanus simplicius Plain Longtail Urbanus procne Brown Longtail -

Section 2. Jack Pine (Pinus Banksiana)
SECTION 2. JACK PINE - 57 Section 2. Jack pine (Pinus banksiana) 1. Taxonomy and use 1.1. Taxonomy The largest genus in the family Pinaceae, Pinus L., which consists of about 110 pine species, occurs naturally through much of the Northern Hemisphere, from the far north to the cooler montane tropics (Peterson, 1980; Richardson, 1998). Two subgenera are usually recognised: hard pines (generally with much resin, wood close-grained, sheath of a leaf fascicle persistent, two fibrovascular bundles per needle — the diploxylon pines); and soft, or white pines (generally little resin, wood coarse-grained, sheath sheds early, one fibrovascular bundle in a needle — the haploxylon pines). These subgenera are called respectively subg. Pinus and subg. Strobus (Little and Critchfield, 1969; Price et al., 1998). Occasionally, one to about half the species (20 spp.) in subg. Strobus are classified instead in a variable subg. Ducampopinus. Jack pine (Pinus banksiana Lamb.) and its close relative lodgepole pine (Pinus contorta Dougl. Ex Loud.) are in subg. Pinus, subsection Contortae, which is classified either in section Trifoliis or a larger section Pinus (Little and Critchfield, 1969; Price et al., 1998). Additionally, subsect. Contortae usually includes Virginia pine (P. virginiana) and sand pine (P. clausa), which are in southeastern USA. Jack pine has two quite short (2-5 cm) stiff needles per fascicle (cluster) and lopsided (asymmetric) cones that curve toward the branch tip, and the cone scales often have a tiny prickle at each tip (Kral, 1993). Non-taxonomic ecological or biological variants of jack pine have been described, including dwarf, pendulous, and prostrate forms, having variegated needle colouration, and with unusual branching habits (Rudolph and Yeatman, 1982). -

Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; Download Unter
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Entomofauna Jahr/Year: 2007 Band/Volume: 0028 Autor(en)/Author(s): Yefremova Zoya A., Ebrahimi Ebrahim, Yegorenkova Ekaterina Artikel/Article: The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) 321-356 ©Entomofauna Ansfelden/Austria; download unter www.biologiezentrum.at Entomofauna ZEITSCHRIFT FÜR ENTOMOLOGIE Band 28, Heft 25: 321-356 ISSN 0250-4413 Ansfelden, 30. November 2007 The Subfamilies Eulophinae, Entedoninae and Tetrastichinae in Iran, with description of new species (Hymenoptera: Eulophidae) Zoya YEFREMOVA, Ebrahim EBRAHIMI & Ekaterina YEGORENKOVA Abstract This paper reflects the current degree of research of Eulophidae and their hosts in Iran. A list of the species from Iran belonging to the subfamilies Eulophinae, Entedoninae and Tetrastichinae is presented. In the present work 47 species from 22 genera are recorded from Iran. Two species (Cirrospilus scapus sp. nov. and Aprostocetus persicus sp. nov.) are described as new. A list of 45 host-parasitoid associations in Iran and keys to Iranian species of three genera (Cirrospilus, Diglyphus and Aprostocetus) are included. Zusammenfassung Dieser Artikel zeigt den derzeitigen Untersuchungsstand an eulophiden Wespen und ihrer Wirte im Iran. Eine Liste der für den Iran festgestellten Arten der Unterfamilien Eu- lophinae, Entedoninae und Tetrastichinae wird präsentiert. Mit vorliegender Arbeit werden 47 Arten in 22 Gattungen aus dem Iran nachgewiesen. Zwei neue Arten (Cirrospilus sca- pus sp. nov. und Aprostocetus persicus sp. nov.) werden beschrieben. Eine Liste von 45 Wirts- und Parasitoid-Beziehungen im Iran und ein Schlüssel für 3 Gattungen (Cirro- spilus, Diglyphus und Aprostocetus) sind in der Arbeit enthalten. -

Pollinators Full.Pdf
Hymenoptera: Bees Hymenoptera: Bees Hymenoptera: Wasps, Ants & Sawies Hymenoptera: Wasps, Ants & Sawies Pollinator Insects of the South West Slopes of NSW and North East Victoria This guide has been prepared to aid identication of a Pollinator Insects selection of common pollinator insects. Insects Pollinator This guide provides a good starting point, but many species can look similar. Please see the references and websites listed if you would like help with accurate of the South West Slopes of NSW species identification. and North East Victoria An identification and conservation guide Halictid bee Hylaeus bee Gasteruptiid wasp Hairy ower wasp Halictidae Colletidae Gasteruptiidae Scoliidae of the South West Slopes NSW and North East Victoria Blue-banded bee Chequered cuckoo bee Ant Cream-spotted ichneumon wasp Apidae Apidae Formicidae Ichnuemonidae Hylaeus bee (bubbling) Large Lasioglossum sp. Orange ichneumon wasp Paper wasp Colletidae Halictidae Ichnuemonidae Vespidae Orange ichneumon wasp Ichnuemonidae Online pollinator information resources Aussie Bee aussiebee.com.au Bee Aware Australia beeawareaustralia.org Common spring bee European honey bee Cuckoo wasp European wasp Australian Museum Plant2pollinator Colletidae Apidae Chrysididae Vespidae australianmuseum.net.au/welcome-to-plant2pollinator PaDIL Australian Pollinators padil.gov.au/pollinators Bowerbird bowerbird.org.au Leafcutter bee Red bee Paper wasp Sawy adult Victorian butteries Megachilidae Halictidae Vespidae Tenthredinidae museumvictoria.com.au/bioinformatics/butter/images/bthumbmenu.htm Atlas of Living Australia ala.org.au Hymenoptera: Bees Hymenoptera: Wasps, Ants & Sawies Wild Pollinator Count wildpollinatorcount.com • Around 2,000 native bee species currently known. • Around 8,000 native species currently known; many more undescribed. Photography • Mostly found in sunny, open woodlands, gardens and meadows with lots • Found in all habitats. -

Unexpectedly High Levels of Parasitism of Wheat Stem Sawfly Larvae in Postcutting Diapause Chambers Author(S) :Tatyana A
Unexpectedly High Levels of Parasitism of Wheat Stem Sawfly Larvae in Postcutting Diapause Chambers Author(s) :Tatyana A. Rand, Debra K. Waters, Thomas G. Shanower Source: The Canadian Entomologist, 143(5):455-459. 2011. Published By: Entomological Society of Canada URL: http://www.bioone.org/doi/full/10.4039/n11-023 BioOne (www.bioone.org) is a nonprofit, online aggregation of core research in the biological, ecological, and environmental sciences. BioOne provides a sustainable online platform for over 170 journals and books published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Web site, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/page/ terms_of_use. Usage of BioOne content is strictly limited to personal, educational, and non-commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. 455 Unexpectedly high levels of parasitism of wheat stem sawfly larvae in postcutting diapause chambers Tatyana A. Rand, Debra K. Waters, Thomas G. Shanower Abstract*We examined rates of late-season parasitism of larvae of the wheat stem sawfly, Cephus cinctus Norton (Hymenoptera: Cephidae), by native species of Bracon F. (Hymenop- tera: Braconidae) over 8 years in Montana and North Dakota, United States of America. We found that rates of parasitism of larvae in diapause chambers reached a maximum of 46%, exceeding the previously reported maximum of 2.5% in 75% of sites and years examined. -
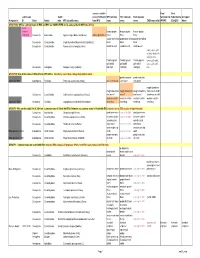
Common Names Working-2008Jan
correct scientific Final Final submission Subfa name (if different WFI common ESC common ESA common Submitted to Submitted to Accepted Assigned to: ID Order Family mily WFI scientific name from WFI) name name name CABI name info? WFIWC: ESA/ESC: Name GROUP 000: Official common name in ESA and ESC are COMPLETED and is same as that in WFI (take off list) need change? bronze poplar bronze poplar bronze poplar 1026-Bup Coleoptera Buprestidae Agrilus liragus Barter and Brown Agrilus granulatus borer borer borer poplar-and-willow poplar-and-willow poplar-and-willow Coleoptera Curculionidae Cryptorhynchus [Sternochetus] lapathi (L.) borer borer borer Coleoptera Curculionidae Nemocestes incomptus (Horn) woods weevil woods weevil wood weevil cooley spruce gall adelgid; douglas fir adelges; sitka Cooley spruce Cooley spruce Cooley spruce spruce gall aphid; gall aphid gall aphid gall aphid spruce gall aphid, Homoptera Adelgidae Adelges cooleyi (Gillette) (adelgid) (adelgid) (adelgid) blue GROUP 00: One of the names in ESA, ESC or WFI differs (Decide by case; likely change highlighted name) ponderosa pine ponderosa pine approved 1989 Lepidoptera Pyralidae Dioryctria auranticella (Grote) pine coneworm coneworm coneworm rough strawberry rough strawberry rough strawberry rough strawberry root weevil; rough Coleoptera Curculionidae Otiorhynchus rugosostriatus (Goeze) root weevil weevil root weevil strawberry weevil western conifer western conifer- western conifer- western conifer- approved 1989 Hemiptera Coreidae Leptoglossus occidentalis Heidemann seed -

Parasitoid Complex of Overwintering Cocoons of Neodiprion Huizeensis (Hymenoptera: Diprionidae) in Guizhou, China
Revista Colombiana de Entomología 42 (1): 43-47 (Enero - Junio 2016) 43 Parasitoid complex of overwintering cocoons of Neodiprion huizeensis (Hymenoptera: Diprionidae) in Guizhou, China Complejo de parasitoides de capullos invernales de Neodiprion huizeensis (Hymenoptera: Diprionidae) en Guizhou, China LI TAO1,2, SHENG MAO-LING1,3, SUN SHU-PING1,4 and LUO YOU-QING5 Abstract: The conifer sawfly, Neodiprion huizeensis (Hymenoptera: Diprionidae), is an injurious leaf feeder of Pinus spp. (Pinaceae) in China. Its parasitoid complex of overwintering cocoons was investigated in Weining, Guizhou during 2012. The average parasitism rate of overwintering cocoons of N. huizeensis by the parasitoid complex was 34.6%. The parasitoid complex included Drino auricapita (Diptera: Tachinidae), ichneumonids, and Trichomalus sp. (Hymenoptera: Pteromalidae). The average parasitism rate of N. huizeensis by D. auricapita was 13.1%. The puparial period of D. auricapita averaged 16.4 ± 0.1 d. The female to male ratio was 1.1: 1. The ichneumonid complex included Aptesis grandis, A. melana, A. nigricoxa, Delomerista indica, Lamachus rufiabdominalis, L. nigrus, Bathythrix sp., Caenocryptus sp., Exyston spp., Gelis sp., Goryphus sp., and Olesicampe sp. The parasitism rate of N. huizeensis by ichneumonids was 17.1%. The parasitism rate of N. huizeensis by Trichomalus sp. was 4.5%, and the female to male ratio was 3.7: 1. The dominant species of parasitoids was D. auricapita followed by A. melana. The emergence of overwintered adults of N. huizeensis had two peaks: the first from the 17th to the 23rd of February, 2012; the second from February 29th to March 15th, 2012. The emergence of the parasitoid complexes coincided with each other and occurred from February 23rd to March 6th, 2012. -

Supplementary Materials Neodiprion Sawflies
Supplementary Materials Neodiprion sawflies: life history description and utility as a model for parent-offspring conflict To provide context for our comparative analysis of Neodiprion clutch-size traits, we provide relevant life history details (reviewed in Coppel and Benjamin 1965; Knerer and Atwood 1973; Wilson et al. 1992; Knerer 1993). Adult females emerge from cocoons with a full complement of mature eggs, find a suitable host, and attract males via a powerful pheromone. Shortly after mating, females use their saw-like ovipositors to embed their eggs within pine needles. While females of some species tend to lay their full complement of eggs on a single branch terminus, females of other species seek out multiple branches or trees for oviposition. Overall, female oviposition behavior is highly species-specific and, in some cases, diagnostic (Ghent 1959). Adult Neodiprion are non-feeding and short-lived (~2-4 days), dying soon after mating and oviposition. After hatching from eggs, Neodiprion larvae of many species form feeding aggregations that remain intact to varying degrees across 4-7 feeding instars, depending on the sex and the species. As larvae defoliate pine branches, they migrate to new branches and sometimes to new host trees (Benjamin 1955, Smirnoff 1960). During these migrations, colonies may undergo fission and fusion events (Codella and Raffa 1993, Codella and Raffa 1995, Costa and Loque 2001). Thus, while initial colony size corresponds closely to egg-clutch size, larvae are highly mobile and their dispersal behavior has the potential to substantially alter colony size (Codella and Raffa 1995). Beyond having a variable and well-documented natural history, Neodiprion provides an excellent test case for examining coevolution of female egg-laying and larval grouping behaviors because, as is likely the case for many insects, feeding in groups could confer both costs and benefits to the larvae (Codella and Raffa 1995, Heitland and Pschorn-Walcher 1993).