Seed Biology 95 Fig
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Biomimética De Estructuras Vegetales Mejorando La Seguridad En El Ciclismo a Partir De La Olla De Mono
BIOMIMÉTICA DE ESTRUCTURAS VEGETALES MEJORANDO LA SEGURIDAD EN EL CICLISMO A PARTIR DE LA OLLA DE MONO Lucía Paulina Buelvas Álvarez Jenny Alexandra Ramírez Osorio Universidad Pontificia Bolivariana Escuela de Arquitectura y Diseño Facultad de Diseño Industrial Medellín 2014 Línea de morfología experimental CONTENIDO Resumen – Abstract Introducción Planteamiento del proyecto. Tema general del proyecto. Características generales del proyecto de investigación. Problema identifica. Elementos del problema. Hipótesis o pregunta que se formula frente al tema como planteamiento de la investigación. Justificación del proyecto. Validez del proyecto en el contexto de la investigación en diseño industrial. Oportunidades que representa para el desarrollo de nuevos productos o estrategias. Objetivos. Objetivo general. Objetivos específicos. Alcance. Límites y metas temáticas para el proyecto. Límites y metas metodológicas para el proyecto. Marco de referencia. Antecedentes. Estado del arte. Conceptualización de los elementos del problema. Materiales y métodos Material botánico Métodos Caracterización anatómica y morfológica del fruto del árbol Olla de mono Identificación del tipo de materiales del fruto de la Olla de mono Definición del nivel de desempeño funcional estructural del fruto Resultados y discusión Conclusiones Agradecimientos Glosario Bibliografía Biomimética de estructuras vegetales Mejorando la seguridad en el ciclismo a partir de la Olla de mono Lucía Paulina Buelvas Álvarez – Jenny Alexandra Ramírez Osorio Universidad Pontificia Bolivariana, Medellín, Colombia “El éxito parece ser en buena parte cuestión de perseverar después de que otros hayan abandonado.” William Feather Resumen Actualmente el ciclismo es uno de los deportes con mayor nivel de aceptación en Colombia. Para su práctica se requiere del uso de sistemas de protección personal que garanticen la seguridad del deportista, siendo el casco uno de los objetos más importantes de este equipamiento. -
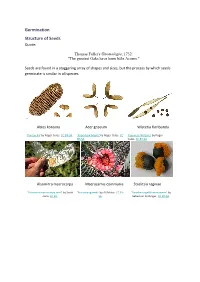
Germination Structure of Seeds Quote
Germination Structure of Seeds Quote: Thomas Fuller's Gnomologia, 1732: "The greatest Oaks have been little Acorns." Seeds are found in a staggering array of shapes and sizes, but the process by which seeds germinate is similar in all species. Abies koreana Acer griseum Wisteria floribunda 'Korean Fir' by Roger Culos. CC BY-SA. 'Paperbark Maple' by Roger Culos. CC 'Japanese Wisteria' by Roger BY-SA. Culos. CC BY-SA. Alsomitra macrocarpa Macrozamia communis Strelitzia reginae 'Alsomitra macrocarpa seed' by Scott 'BurrawangSeeds' by AYArktos. CC BY- 'Paradiesvogelblumensamen' by Zona. CC BY. SA. Sebastian Stabinger. CC BY-SA. Taraxicum officinale Stephanotis floribunda Phleum pratense 'Achane of Taraxacum sect. 'Stephanotis seed' by L. Marie"/Lenore 'Timoteegras vruchten Phleum Ruderalia' by Didier Edman, Sunnyvale, CA. CC BY. pratense' by Rasbak. CC BY-SA. Descouens. CC BY-SA. Dicotyledon seeds testa epicotyl plumule hypocotyl cotyledon radicle 'Aesculus hippocastanum seed section' by Boronian. CC BY. plumule epicotyl hypocotyl testa hilum radicle cotyledon micropyle endosperm Monocotyledon seeds endosperm epicotyl testa hypocotyl cotyledon radicle Parts of a seed Testa The seed coat. A protective layer which is tough and hard and it protects the seed from attack by insects, fungi and bacteria. Cotyledon Dicotyledons have 2 cotyledons Monocotyledons have 1 cotyledon A cotyledon is an embryonic leaf. It is the first leaf to appear when a seedling grows. They often contain reserves of food which the developing seedling can use to grow. Epicotyl The section of stem between the cotyledon(s) and the plumule. In a seedling it is the section of stem between the cotyledons and the first true leaves. -

Diversity and Distributional Patterns of Aroids (Alismatales: Araceae) Along an Elevational Gradient in Darién, Panama
Webbia Journal of Plant Taxonomy and Geography ISSN: 0083-7792 (Print) 2169-4060 (Online) Journal homepage: https://www.tandfonline.com/loi/tweb20 Diversity and distributional patterns of aroids (Alismatales: Araceae) along an elevational gradient in Darién, Panama Orlando O. Ortiz, María S. de Stapf & Thomas B. Croat To cite this article: Orlando O. Ortiz, María S. de Stapf & Thomas B. Croat (2019): Diversity and distributional patterns of aroids (Alismatales: Araceae) along an elevational gradient in Darién, Panama, Webbia, DOI: 10.1080/00837792.2019.1646465 To link to this article: https://doi.org/10.1080/00837792.2019.1646465 View supplementary material Published online: 28 Aug 2019. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tweb20 WEBBIA https://doi.org/10.1080/00837792.2019.1646465 ARTICLE Diversity and distributional patterns of aroids (Alismatales: Araceae) along an elevational gradient in Darién, Panama Orlando O. Ortiz a, María S. de Stapfa,b and Thomas B. Croatc aHerbario PMA, Universidad de Panamá, Estafeta Universitaria, Panama City, Panama; bDepartamento de Botánica, Universidad de Panamá, Estafeta Universitaria, Panama City, Panama; cDepartment of Research (Monographs Section), Missouri Botanical Garden, St. Louis, MO, USA ABSTRACT ARTICLE HISTORY The family Araceae (aroids) represents an ecologically important and diverse group of plants in Received 24 May 2019 Panama, represented by 25 genera, 615 species, of which 277 (45%) are considered endemic. Accepted 18 July 2019 The aim of this study is to analyse the diversity and distributional patterns of aroids along an KEYWORDS fi elevation gradient in the species-rich forests of Darién, Panama. -

Drupe. Fruit with a Hard Endocarp (Figs. 67 and 71-73); E.G., and Sterculiaceae (Helicteres Guazumaefolia, Sterculia)
Fig. 71. Fig. 72. Fig. 73. Drupe. Fruit with a hard endocarp (figs. 67 and 71-73); e.g., and Sterculiaceae (Helicteres guazumaefolia, Sterculia). Anacardiaceae (Spondias purpurea, S. mombin, Mangifera indi- Desmopsis bibracteata (Annonaceae) has aggregate follicles ca, Tapirira), Caryocaraceae (Caryocar costaricense), Chrysobal- with constrictions between successive seeds, similar to those anaceae (Licania), Euphorbiaceae (Hyeronima), Malpighiaceae found in loments. (Byrsonima crispa), Olacaceae (Minquartia guianensis), Sapin- daceae (Meliccocus bijugatus), and Verbenaceae (Vitex cooperi). Samaracetum. Aggregate of samaras (fig. 74); e.g., Aceraceae (Acer pseudoplatanus), Magnoliaceae (Liriodendron tulipifera Hesperidium. Septicidal berry with a thick pericarp (fig. 67). L.), Sapindaceae (Thouinidium dodecandrum), and Tiliaceae Most of the fruit is derived from glandular trichomes. It is (Goethalsia meiantha). typical of the Rutaceae (Citrus). Multiple Fruits Aggregate Fruits Multiple fruits are found along a single axis and are usually coalescent. The most common types follow: Several types of aggregate fruits exist (fig. 74): Bibacca. Double fused berry; e.g., Lonicera. Achenacetum. Cluster of achenia; e.g., the strawberry (Fra- garia vesca). Sorosis. Fruits usually coalescent on a central axis; they derive from the ovaries of several flowers; e.g., Moraceae (Artocarpus Baccacetum or etaerio. Aggregate of berries; e.g., Annonaceae altilis). (Asimina triloba, Cananga odorata, Uvaria). The berries can be aggregate and syncarpic as in Annona reticulata, A. muricata, Syconium. Syncarp with many achenia in the inner wall of a A. pittieri and other species. hollow receptacle (fig. 74); e.g., Ficus. Drupacetum. Aggregate of druplets; e.g., Bursera simaruba THE GYMNOSPERM FRUIT (Burseraceae). Fertilization stimulates the growth of young gynostrobiles Folliacetum. Aggregate of follicles; e.g., Annonaceae which in species such as Pinus are more than 1 year old. -

The Effect of Seed Weight on Photosynthetic Area Development and Weight of the Sainfoin (Onebrychis Spp
The effect of seed weight on photosynthetic area development and weight of the sainfoin (Onebrychis spp. Scop.) seedling by Stephen Carl Fransen A thesis submitted in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Agronomy Montana State University © Copyright by Stephen Carl Fransen (1975) Abstract: The hypothesis that cotyledon area to seed weight ratio might vary among sainfoin accessions was tested by comparing regression coefficients of area on weight. In addition, effects of seed weight upon areas and weights of cotyledons, first and second leaves and total seedlings were studied. Ten accessions of sainfoin representing three different species were studied. After 17 days of growth in a growth chamber, seedlings of all accessions were sampled for the characteristics to be studied. The ratios of cotyledon area to seed weight were similar for most accessions. Seedlings from heavier seeds emerged and developed more rapidly than seedlings from lighter seeds. Embryo axis length and width and leaf primordia length were all highly correlated with seed weight. Areas and weights of cotyledons, first and second leaves were correlated with seed weight for most accessions. Total seedling area and weight, at 17 days of age, were also correlated with initial seed weight. Trifoliolate seedlings had greater first leaf area but less second leaf area than unifoliolate seedlings. As a result, trifoliolate seedlings had only 3.5 percent more total leaf area than unifoliolate seedlings at 17 days. These results help explain some of the variability among previous tests comparing unifoliolate and trifoliolate seedlings for seedling vigor. STATEMENT OF PERMISSION TO COPY Iu presenting this thesis in partial fulfillment of the requirements for an advanced degree at Montana State University, I agree that the Library shall make it freely .available for inspection„ I further agree that permission for extensive copying of this thesis for scholarly purposes- may be granted by my major professor, or, in his absence, by the Director of Libraries. -

A Global Perspective on the Origins of Agriculture: the Importance of Unconscious Selection
A global perspective on the origins of agriculture: the importance of unconscious selection Thomas Kluyver Department of Animal and Plant Sciences A thesis submitted for the degree of Doctor of Philosophy July 2013 1 Acknowledgements My primary supervisor, Colin Osborne, has provided advice, encouragement and inspiration throughout my PhD. My supervisors in the Department of Archaeology, Glynis Jones and Mike Charles, have patiently helped me to get to grips with a field which I had never studied before this project. Mark Rees’ advice about statistics has also been invaluable. I am grateful to Irene Johnson, for her eminently practical help with growing all kinds of plants, and to Emily Mockford and Chris Bennett, for painstakingly dissecting beet seed capsules to weigh individual seeds. Katherine Haynes and Rebecca Crabtree weighed seed of modern garden vegetables for chapter 3, and that chapter also could not have been written without people and organisations around the world who shared their data with me, including Benoît Pujol (Laboratoire Évolution et Diversité Biologique, France), the Botanical Information Section at RBG Kew, the USDA National Genetic Resources Program, the International Potato Centre (CIP) in Peru, and EMBRAPA in Brazil. Over the last few years, I have enjoyed a warm, friendly and intellectually stimulating environment in Sheffield. It has been a pleasure to work with the people in Colin Osborne’s lab group, as well as the many others who I have got to know. My PhD research was funded by a university studentship from the University of Sheffield, for which I am very thankful. Last but not least, my thanks to my girlfriend and my family, for their support both during my PhD and in the years of education which prepared me to undertake it. -

Early Soybean Growth and Development
EARLY SOYBEAN GROWTH AND DEVELOPMENT A soybean seed has two distinct parts: the cotyledons and the embryo. The two cotyledons are the main food storage structure, which supply food during emergence and for the seven to ten days after emergence through the V1 growth stage. The embryo itself is comprised of three parts. 1. The radicle is the first root and it’s the first part of the embryo to penetrate the seed coat. Lateral roots form from the radicle, and tiny root hairs then develop on the lateral roots. Root hairs are the main absorbing surface of the entire root system. A soybean’s root system branches and rebranches within the first four to five weeks after planting, with the bulk of root mass persisting in the top six inches of soil. 2. The hypocotyl is the stem below the cotyledon. It begins to elongate after the radicle and forms an arch, which is pushed upward. As the arch breaks the soil surface, it pulls the cotyledons and epicotyl up. The uppermost cells of the hypocotyl stop growing as cells on the underside continue to straighten the arch. This process lifts the cotyledons into an upright position. 3. The epicotyl is comprised of the small leaves, stem and the apical growing point. The epicotyl is protected between the cotyledons until after emergence. The loss of a cotyledon is not lethal to the seedling, but damage to the epicotyl is. The hypocotyl arch is a large structure that is relative to seed size, utilizing considerable seed energy to push through the soil. -

Agricultural Marketing Service, USDA § 201.56–10
Agricultural Marketing Service, USDA § 201.56–10 (A) One or more essential structures and become thin, leaf-like, and photo- impaired as a result of decay from pri- synthetic. mary infection. (3) Shoot system: The hypocotyl (B) Albino. elongates carrying the cotyledons above the soil surface. The epicotyl [59 FR 64504, Dec. 14, 1994] usually does not show any development § 201.56–8 Flax family, Linaceae. within the test period. Areas of yel- lowish pigmentation may develop on Kind of seed: Flax. the hypocotyl in cotton. (a) General description. (4) Root system: A primary root, with (1) Germination habit: Epigeal dicot. secondary roots usually developing (Due to the mucilaginous nature of the within the test period. Areas of yel- seed coat, seedlings germinated on lowish pigmentation may develop on blotters may adhere to the blotter and the root in cotton. appear to be negatively geotropic.) (b) Abnormal seedling description. (2) Food reserves: Cotyledons which (1) Cotyledons: expand and become photosynthetic. (i) Less than half of the original cot- (3) Shoot system: The hypocotyl yledon tissue remaining attached. elongates carrying the cotyledons (ii) Less than half of the original cot- above the soil surface. The epicotyl yledon tissue free of necrosis or decay. usually does not show any development (Remove any attached seed coats at within the test period. the end of the test period for evalua- (4) Root system: A primary root, with tion of cotyledons.) secondary roots usually developing (2) Epicotyl: within the test period. (i) Missing. (May be assumed to be (b) Abnormal seedling description. present if both cotyledons are intact.) (1) Cotyledons: (ii) [Reserved] (i) Less than half of the original cot- (3) Hypocotyl: yledon tissue remaining attached. -

Leguminosae: Mimosoideae) Com Ênfase Em Calliandra Benth
ELVIA RODRIGUES DE SOUZA ESTUDOS FILOGENÉTICOS NA TRIBO INGEAE (LEGUMINOSAE: MIMOSOIDEAE) COM ÊNFASE EM CALLIANDRA BENTH. E GÊNEROS AFINS Feira de Santana – BA 2007 UNIVERSIDADE ESTADUAL DE FEIRA DE SANTANA DEPARTAMENTO DE CIÊNCIAS BIOLÓGICAS PROGRAMA DE PÓS-GRADUAÇÃO EM BOTÂNICA ESTUDOS FILOGENÉTICOS NA TRIBO INGEAE (LEGUMINOSAE: MIMOSOIDEAE) COM ÊNFASE EM CALLIANDRA BENTH. E GÊNEROS AFINS ÉLVIA RODRIGUES DE SOUZA Tese apresentada ao Programa de Pós-Graduação em Botânica da Universidade Estadual de Feira de Santana como parte dos requisitos para a obtenção do título de Doutora em Botânica. ORIENTADOR: PROF. DR. LUCIANO PAGANUCCI DE QUEIROZ CO- ORIENTADOR: PROF. DR. CÁSSIO VAN DEN BERG FEIRA DE SANTANA – BA 2007 Ficha catalográfica: Biblioteca Central Julieta Carteado Souza, Élvia Rodrigues de S714e Estudos filogenéticos na tribo Ingeae (Leguminosae: Mimosoideae) com ênfase em Calliandra Benth. e gêneros afins / Élvia Rodrigues de Souza. – Feira de Santana, 2007. 110 f. : il. Orientador: Luciano Paganucci de Queiroz Co-orientador: Cássio van den Berg Tese (Doutorado em Botânica)– Departamento de Ciências Biológicas, Universidade Estadual de Feira de Santana, 2007. 1. Leguminosae. 2. Mimosoideae. 3. Calliandra. I. Queiroz, Luciano Paganucci. II. Berg, Cássio van den. III. Universidade Estadual de Feira de Santana. IV. Departamento de Ciências Biológicas. V. Título. CDU: 582.736/.737 “Existe somente uma idade para a gente ser feliz, somente uma época na vida de cada pessoa em que é possível sonhar e fazer planos e ter energia bastante para realizá-los a despeito de todas as dificuldades e obstáculos. Uma só idade para a gente se encantar com a vida e viver apaixonadamente e desfrutar tudo com toda intensidade sem medo nem culpa de sentir prazer. -

1 Seed Storage, Germination, Quality, and Enhancements
1 Seed Storage, Germination, Quality, and Enhancements Alan G. Taylor School of Integrative Plant Science, Horticulture Unit, Cornell AgriTech, Cornell University, Geneva, New York 14456, USA Before vegetables are harvested, before their The focus of this chapter is on postharvest growth and development, even before the seed- aspects of seeds; the period of seed development ling becomes photosynthetically competent, the and production stages are not addressed. We start source of the vegetable—the seed—holds major with storage of seeds with low moisture content keys to final product yield. To fully comprehend as most vegetable crop seeds have unique char- the complexities of vegetable crop growth and acteristics that permit them to withstand desic- development, we must understand seed physi- cation (Leopold and Vertucci, 1986). Desiccation ology related to storage, germination, quality, tolerance is essential for long-term survival and and enhancements. The challenge is to present allows for a time interval between seed produc- an in-depth knowledge of vegetable seeds that tion and crop production. Most vegetable seeds covers considerable diversity with respect to bo- imbibe water readily and, provided with a suit- tanical classification, seed size, and composition. able environment, will germinate and resume This chapter and book encompasses 33 common active growth. The seed uses its reserve materials vegetable crop seeds from ten plant families following germination and then becomes an (Table 1.1). This diverse group of plants makes a active photosynthetic seedling, fixing its own comprehensive overview of vegetable seeds diffi- carbon and producing energy. cult to achieve, especially since there is little sci- Seed quality is a broad term and encom- entific literature on seed physiology of many passes several attributes of seeds including the small-seeded crops of minor economic import- germination and seedling performance. -

Flower Speciesspecies
13 June 2011 ISTA GERMINATION SEMINAR GERMINATIONGERMINATION CHARACTERISTICSCHARACTERISTICS OFOF FLOWERFLOWER SPECIESSPECIES RITA ZECCHINELLI - ISTA FLOWER SEED TESTING COMMITTEE Luca Flower species in the ISTA Rules: . 352 species . 192 genera . 55 families 13 June 2011 ISTA GERMINATION SEMINAR RITA ZECCHINELLI DICOTYLEDON FAMILIES Family N°genera N°species Family N°genera N°species Acanthaceae 1 1 Hypericaceae 1 1 Aizoaceae 1 1 Iridaceae 1 1 Amaranthaceae 3 6 Lamiaceae 14 21 Apiaceae 3 4 Linaceae 1 4 Apocynaceae 1 1 Malvaceae 6 7 Araliaceae 2 2 Nyctaginaceae 1 1 Asclepiadeaceae 1 1 Onograceae 2 4 Asteraceae 49 86 Papaveraceae 3 7 MONOCOTYLEDON FAMILIES Balsaminaceae 1 2 Plumbaginaceae 4 8 Family N°genera N°species Begoniaceae 1 2 Polemoniaceae 3 5 Amaryllidaceae 1 1 Boraginaceae 6 10 Polygonaceae 1 1 Asparagaceae 1 2 Brassicaceae 11 22 Portulaceae 1 1 Asphodelaceae 1 1 Campanulaceae 3 13 Primulaceae 3 12 Liliaceae 1 1 Capparidaceae 1 1 Proteaceae 1 1 Poaceae 2 2 Caryophyllaceae 6 16 Ranuncolaceae 7 20 Chenopodiaceae 1 1 Resedaceae 1 1 Cistaceae 1 1 Rosaceae 2 3 Convolvulaceae 2 5 Rutaceae 1 1 Crassulaceae 1 3 Saxifragaceae 1 1 Dipsacaceae 1 2 Scrophulariaceae 10 22 Fabaceae 4 8 Solanaceae 9 15 Gentianaceae 1 1 Tropaeolaceae 1 3 Geraniaceae 2 2 Valerianceae 1 1 Gesneriaceae 2 2 Verbenaceae 2 4 Hydrophyllaceae 3 4 Violaceae 1 3 13 June 2011 ISTA GERMINATION SEMINAR RITA ZECCHINELLI What flower species have in common? . ornamental use . fast changing trends in the market . for seed testing laboratories - less experience in standardized -

Endorsement and Phylogenetic Analysis of Some Fabaceae Plants Based
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.27.454001; this version posted July 28, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 Endorsement and Phylogenetic Analysis of some Fabaceae Plants based 2 on DNA Barcoding gene MatK 3 Nader R. Abdelsalam1, *, Mohamed E. Hasan2, **, Samar M.A. Rabie1 , Houssam El- 4 Din M.F. El-wakeel 1, Amera F. Zaitoun 1 , Rehab Y. Ghareeb3, Aly Z. Abdelsalam 4, 5 Hesham M. Aly 5 , Amira A. Ibrahim3, Alaa A. Hemeida2 6 1 Agricultural Botany Department, Faculty of Agriculture, Saba Basha, Alexandria 7 University, Alexandria, 21531 Egypt. 8 2 Bioinformatics Department, Genetic Engineering and Biotechnology Research Institute, 9 University of Sadat City. 10 3Plant Protection and Biomolecular Diagnosis Department, Arid Lands Cultivation 11 Research Institute (ALCRI), City of Scientific Research and Technological Applications 12 (SARTA, City), New Borg El Arab City, Alexandria 21934, Egypt. 13 4 Genetics Department, Faculty of Agriculture, Ain-Shams University, Cairo 11566, Egypt. 14 5 Department of Forestry and Wood Technology, Horticulture Institute, Agriculture 15 Research Center, Antoniadis Botanical Garden, Alexandria 21554, Egypt. 16 17 18 19 20 21 22 23 24 25 26 * Correspondence: * [email protected] Tel.: (+20 1066329045) 27 ** [email protected] ; Tel.: (+20 106 170 8284) 28 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.27.454001; this version posted July 28, 2021.