19. Radiation and Optics in the Atmosphere and 19.3 Aerosols and Clouds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Photochemically Driven Collapse of Titan's Atmosphere
REPORTS has escaped, the surface temperature again Photochemically Driven Collapse decreases, down to about 86 K, slightly of Titan’s Atmosphere above the equilibrium temperature, because of the slight greenhouse effect resulting Ralph D. Lorenz,* Christopher P. McKay, Jonathan I. Lunine from the N2 opacity below (longward of) 200 cm21. Saturn’s giant moon Titan has a thick (1.5 bar) nitrogen atmosphere, which has a These calculations assume the present temperature structure that is controlled by the absorption of solar and thermal radiation solar constant of 15.6 W m22 and a surface by methane, hydrogen, and organic aerosols into which methane is irreversibly converted albedo of 0.2 and ignore the effects of N2 by photolysis. Previous studies of Titan’s climate evolution have been done with the condensation. As noted in earlier studies assumption that the methane abundance was maintained against photolytic depletion (5), when the atmosphere cools during the throughout Titan’s history, either by continuous supply from the interior or by buffering CH4 depletion, the model temperature at by a surface or near surface reservoir. Radiative-convective and radiative-saturated some altitudes in the atmosphere (here, at equilibrium models of Titan’s atmosphere show that methane depletion may have al- ;10 km for 20% CH4 surface humidity) lowed Titan’s atmosphere to cool so that nitrogen, its main constituent, condenses onto becomes lower than the saturation temper- the surface, collapsing Titan into a Triton-like frozen state with a thin atmosphere. -

Enhanced Total Internal Reflection Using Low-Index Nanolattice Materials
Letter Vol. 42, No. 20 / October 15 2017 / Optics Letters 4123 Enhanced total internal reflection using low-index nanolattice materials XU A. ZHANG,YI-AN CHEN,ABHIJEET BAGAL, AND CHIH-HAO CHANG* Department of Mechanical and Aerospace Engineering, North Carolina State University, Raleigh, North Carolina 27695, USA *Corresponding author: [email protected] Received 4 August 2017; revised 14 September 2017; accepted 16 September 2017; posted 19 September 2017 (Doc. ID 303972); published 9 October 2017 Low-index materials are key components in integrated Although naturally occurring materials with low refractive photonics and can enhance index contrast and improve per- indices are limited, there are significant research efforts to formance. Such materials can be constructed from porous artificially create low-index materials close to the index of materials, which generally lack mechanical strength and air. These new materials consist of porous structures through are difficult to integrate. Here we demonstrate enhanced to- various conventional fabrication techniques, such as oblique tal internal reflection (TIR) induced by integrating robust deposition [5–9], the sol-gel process [10], and chemical vapor nanolattice materials with periodic architectures between deposition [11]. Due to the air voids, the fabricated porous high-index media. The transmission measurement from structures can have effective refractive indices lower than the the multilayer stack illustrates a cutoff at about a 60° inci- solid material components. However, such materials typically dence angle, indicating an enhanced light trapping effect lack mechanical stability and can induce optical scattering be- through TIR. Light propagation in the nanolattice material cause of the random architectures of the structures. -

Atmospheric Effects Are Looking Up
Atmospheric Effects are Looking Up OASI Workshop 21st May 2018 by Olaf Kirchner Ever seen one of these ? OK, so how about one of these? Atmospheric Effects - caused by sun- or moonlight interacting with liquid water or ice in the air - surprisingly common - always beautiful and one or several phenomena may be seen at the same time - can be in-your-face obvious or very subtle, and ... - ... span the entire sky - a challenge to photograph - very complicated theoretical explanations Effects caused by Liquid Water Droplets - rainbows - glories, Heiligenschein and the Spectre of the Brocken - aureoles / coronae - nacreous / iridescent / Mother-of-Pearl clouds Rainbow Rainbow Ray paths for primary rainbow Ray paths through a spherical water drop Ray paths for secondary rainbow Secondary Rainbow Secondary rainbow Alexander’s Band Supernumerary rainbow Primary rainbow Rainbow Gap in cloud behind observer = partial rainbow Rainbow in spray, Geneva Jet d’Eau Supernumerary Rainbow Interference colours from different lengths of light path Rainbow Circular rainbow seen from an aircraft Rainbows don’t reflect ... Glory Colourful diffraction rings centred on the antisolar point, caused by reflection from spherical droplets Glory ... i.e. centred on where the shadow of your head would be! Brockengespenst = Spectre of the Brocken Taken against fog from Golden Gate Bridge Brocken (1142 m) . Highest point in the Harz mountains Heiligenschein = Halo Antisolar point in hydrothermal steam ... scary stuff Heiligenschein ... i.e. a glory centred on your head -

Using Temperature As the Basis, the Atmosphere Is Divided Into Four Layers
Using temperature as the basis, the atmosphere is divided into four layers. The temperature decrease in the troposphere, the bottom layer in which we live, is called the "environmental lapse rate." Its average value is 6.5°C per kilometer, a figure known as the "normal lapse rate." A temperature "inversion," in which temperatures increase with height, is sometimes observed in shallow layers in the troposphere. The thickness of the troposphere is generally greater in the tropics than in polar regions. Essentially all important weather phenomena occur in the troposphere. Beyond the troposphere lies the stratosphere; the boundary between the troposphere and stratosphere is known as the tropopause. In the stratosphere, the temperature at first remains constant to a height of about 20 kilometers (12 miles) before it begins a sharp increase due to the absorption of ultraviolet radiation from the Sun by ozone. The temperatures continue to increase until the stratopause is encountered at a height of about 50 kilometers (30 miles).In the mesosphere, the third layer, temperatures again decrease with height until the mesopause, some 80 kilometers (50 miles) above the surface.The fourth layer, the thermosphere, with no well-defined upper limit, consists of extremely rarefied air. Temperatures here increase with an increase in altitude.Besides layers defined by vertical variations in temperature, the atmosphere is often divided into two layers based on composition. The homosphere (zone of homogeneous composition), from Earth’s surface to an altitude of about 80 kilometers (50 miles), consists of air that is uniform in terms of the proportions of its component gases. -

Light, Color, and Atmospheric Optics
Light, Color, and Atmospheric Optics GEOL 1350: Introduction To Meteorology 1 2 • During the scattering process, no energy is gained or lost, and therefore, no temperature changes occur. • Scattering depends on the size of objects, in particular on the ratio of object’s diameter vs wavelength: 1. Rayleigh scattering (D/ < 0.03) 2. Mie scattering (0.03 ≤ D/ < 32) 3. Geometric scattering (D/ ≥ 32) 3 4 • Gas scattering: redirection of radiation by a gas molecule without a net transfer of energy of the molecules • Rayleigh scattering: absorption extinction 4 coefficient s depends on 1/ . • Molecules scatter short (blue) wavelengths preferentially over long (red) wavelengths. • The longer pathway of light through the atmosphere the more shorter wavelengths are scattered. 5 • As sunlight enters the atmosphere, the shorter visible wavelengths of violet, blue and green are scattered more by atmospheric gases than are the longer wavelengths of yellow, orange, and especially red. • The scattered waves of violet, blue, and green strike the eye from all directions. • Because our eyes are more sensitive to blue light, these waves, viewed together, produce the sensation of blue coming from all around us. 6 • Rayleigh Scattering • The selective scattering of blue light by air molecules and very small particles can make distant mountains appear blue. The blue ridge mountains in Virginia. 7 • When small particles, such as fine dust and salt, become suspended in the atmosphere, the color of the sky begins to change from blue to milky white. • These particles are large enough to scatter all wavelengths of visible light fairly evenly in all directions. -

Atmospheric Phenomena by Feist
Atmospheric optical phenomena An introductory guide by Mike Feist Effects caused by water droplets— rainbows and coronae The most well known optical sky effect is the rainbow. This, as most people know, sometimes occurs when the Sun is out and it is raining. To see a rainbow you must stand with your back to the Sun with the raindrops in front of you. It does not have to be raining where you are standing but in the direction that you are looking. The arc of the primary (main) bow is centred on the antisolar point, the spot directly oppo- site the Sun, and has a radius of 42°. The antisolar point is actually centred on the shadow of your head. If the Sun is rising or setting and therefore on the horizon, the primary rainbow will be a complete semi- circle and the top will be 42° up in the sky. If, on the other hand, the Sun is 42° up in the sky, the primary bow will be on the horizon, the top just rising or setting. Con- ventionally the rainbow is said to have John Constable. Hampstead Heath with a Rainbow (1836). seven colours but all we need to remember seen in the spray near waterfalls and artifi- ous forms but with a six-sided shape. They is that, in the primary bow, the red is on cial rainbows can be made using a garden may be as flat hexagonal plates or long the outside and the blue on the inside. Out- hose. Rainbows are one of the easiest opti- hexagonal prisms or as a combination of side the primary bow sometimes there is cal effects to photograph although they the two. -

Project Horizon Report
Volume I · SUMMARY AND SUPPORTING CONSIDERATIONS UNITED STATES · ARMY CRD/I ( S) Proposal t c• Establish a Lunar Outpost (C) Chief of Ordnance ·cRD 20 Mar 1 95 9 1. (U) Reference letter to Chief of Ordnance from Chief of Research and Devel opment, subject as above. 2. (C) Subsequent t o approval by t he Chief of Staff of reference, repre sentatives of the Army Ballistic ~tissiles Agency indicat e d that supplementar y guidance would· be r equired concerning the scope of the preliminary investigation s pecified in the reference. In particular these r epresentatives requested guidance concerning the source of funds required to conduct the investigation. 3. (S) I envision expeditious development o! the proposal to establish a lunar outpost to be of critical innportance t o the p. S . Army of the future. This eva luation i s appar ently shar ed by the Chief of Staff in view of his expeditious a pproval and enthusiastic endorsement of initiation of the study. Therefore, the detail to be covered by the investigation and the subs equent plan should be as com plete a s is feas ible in the tin1e limits a llowed and within the funds currently a vailable within t he office of t he Chief of Ordnance. I n this time of limited budget , additional monies are unavailable. Current. programs have been scrutinized r igidly and identifiable "fat'' trimmed awa y. Thus high study costs are prohibitive at this time , 4. (C) I leave it to your discretion t o determine the source and the amount of money to be devoted to this purpose. -
Apihelion Vs
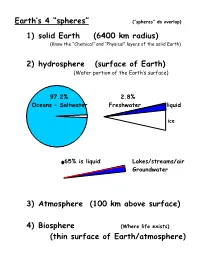
Earth’s 4 “spheres” (“spheres” do overlap) 1) solid Earth (6400 km radius) (Know the “Chemical” and “Physical” layers of the solid Earth) 2) hydrosphere (surface of Earth) (Water portion of the Earth’s surface) 97.2% 2.8% Oceans – Saltwater Freshwater liquid ice .65% is liquid Lakes/streams/air Groundwater 3) Atmosphere (100 km above surface) 4) Biosphere (Where life exists) (thin surface of Earth/atmosphere) Weather vs Climate constantly “average weather” changing 6 basic elements of weather/climate temperature of air humidity of air type & amount of cloudiness type & amount of precipitation pressure exerted by air speed & direction of wind Atmosphere Composition / Ozone Layer (pgs. 6-9) Evolution of Earth’s Atmosphere (pgs. 9-11) Exploring the Atmosphere time line for inventions/discoveries 1593 Galileo “thermometer” 1643 Torricelli barometer 1661 Boyle (P)(V)=constant 1752 Franklin kite -> lightning=electricity 1880(90) manned ballons 1900-today unmanned ballons using radiosondes = radio transmitters that send info on temperature/pressure/relative humidity today rockets & airplanes weather radar & satellites Height/Structure of Atmosphere Exosphere (above 800 km) 100 km 100 km (Ionosphere) 90 km Thermosphere 90 km 80 km 80 km 70 km 70 km 60 km Mesosphere 60 km 50 km 50 km 40 km (Ozone Layer) 40 km 30 km Stratosphere 30 km 20 km 20 km 10 km 10 km Troposphere 0 km 0 km extremely 0o hot really hot 0 100 500 1000 cold Temperature Pressure (mb) Homosphere vs Heterosphere 0-80 km above 80 km uniform distribution varies by mass of molecule N2 O He H Ionosphere located in the Thermosphere/Heterosphere N2 O ionize due to absorbing high-energy solar energy lose electrons and become +charged ions electrons are free to move Solar flares let go of lots of solar energy (charged particles) The charged particles mix with Earth’s magnetic field Charged particles are guided toward N-S magnetic poles Charged particles mix with ionosphere and cause Auroras Electromagnetic Spectrum Seasons are due to angle of sun’s rays. -

Atmospheric Gases and Air Quality
12.3SECTION Atmospheric Gases and Air Quality Key Terms + Exosphere H H criteria air contaminants 500 He- He Ionosphere O Thermosphere 1 1 1 O 1NO 1OZ 1 2 1 1 Heterosphere NZ 1O 90 Photoionization N2 O2 10-5 70 Mesosphere CO 2 Pressure (mmHg) Pressure -3 of O Photodissociation Figure 12.10 Variations in 10 50 (km)Altitude 78% N2 pressure, temperature, and 21% O CO2 O2 Stratosphere 2 the components that make 1% Ar. etc. Ozone layer up Earth’s atmosphere are 30 Homosphere summarized here. 10-1 Infer How can you explain the changes in temperature Troposphere 10 of O Photodissociation H2O with altitude? 1 150 273 300 2000 major major major components components components Temperature (˚C) Figure 12.10 summarizes information about the structure and composition of Earth’s atmosphere. Much of this information is familiar to you from earlier in this unit or from your study of science or geography in earlier grades. As you know from Boyle’s law, gases are compressible. Th us, pressure in the atmosphere decreases with altitude, and this decrease is more rapid at lower altitudes than at higher altitudes. In fact, the vast majority of the mass of the atmosphere—about 99 percent—lies within 30 km of Earth’s surface. About 90 percent of the mass of the atmosphere lies within 15 km of the surface, and about 75 percent lies within 11 km. Th e atmosphere is divided into fi ve distinct regions, based on temperature changes. You may recognize the names of some or perhaps all of these regions: the troposphere, stratosphere, mesosphere, thermosphere, and exosphere. -

Atmospheric Optics
53 Atmospheric Optics Craig F. Bohren Pennsylvania State University, Department of Meteorology, University Park, Pennsylvania, USA Phone: (814) 466-6264; Fax: (814) 865-3663; e-mail: [email protected] Abstract Colors of the sky and colored displays in the sky are mostly a consequence of selective scattering by molecules or particles, absorption usually being irrelevant. Molecular scattering selective by wavelength – incident sunlight of some wavelengths being scattered more than others – but the same in any direction at all wavelengths gives rise to the blue of the sky and the red of sunsets and sunrises. Scattering by particles selective by direction – different in different directions at a given wavelength – gives rise to rainbows, coronas, iridescent clouds, the glory, sun dogs, halos, and other ice-crystal displays. The size distribution of these particles and their shapes determine what is observed, water droplets and ice crystals, for example, resulting in distinct displays. To understand the variation and color and brightness of the sky as well as the brightness of clouds requires coming to grips with multiple scattering: scatterers in an ensemble are illuminated by incident sunlight and by the scattered light from each other. The optical properties of an ensemble are not necessarily those of its individual members. Mirages are a consequence of the spatial variation of coherent scattering (refraction) by air molecules, whereas the green flash owes its existence to both coherent scattering by molecules and incoherent scattering -

Beyond-Line-Of-Sight Communications with Ducting Layer Ergin Dinc, Student Member, IEEE, Ozgur B
IEEE COMMUNICATIONS MAGAZINE 1 Beyond-Line-of-Sight Communications with Ducting Layer Ergin Dinc, Student Member, IEEE, Ozgur B. Akan, Senior Member, IEEE Abstract—Near-surface wave propagation at microwave fre- 8 :JR:`R IQ ].V`V quencies especially 2 GHz and above shows significant depen- dence on atmospheric ducts that are the layer in which rapid decrease in the refractive index occurs. The propagating signals in the atmospheric ducts are trapped between the ducting layer and the sea surface, so that the power of the propagating signals do not spread isotropically through the atmosphere. As a result, these signals have low path-loss and can travel over-the-horizon. V: Since atmospheric ducts are nearly permanent at maritime and coastal environments, ducting layer communication is a promis- 8 IQ ].V`1H%H ing method for beyond-Line-of-Sight (b-LoS) communications especially in naval communications. To this end, we overview %H 1J$:7V` the characteristics and the channel modeling approaches for the ducting layer communications by outlining possible open research areas. In addition, we review the possible utilization of the ducting layer in Network Centric Operations (NCO) to empower decision making for the b-LoS operations. V: Index Terms—Atmospheric ducts, ducting layer, refractivity, beyond-line-of-sight communications Fig. 1. Signal spreading in the standard atmosphere and the atmospheric duct. I. INTRODUCTION TMOSPHERIC ducts that are caused by rapid decrease Ducting layer studies mainly focus on refractivity estimation A in the refractive index of the lower atmosphere have techniques and radar path-loss calculations [1], [2]. However, tremendous effects on the near-surface wave propagation. -

Einstein's Mirage
Paul L. Schechter Einstein’s Mirage he first prediction of Einstein’s general theoryof relativity T to be verified experimentallywas the deflection of light by a massive body—the Sun. In weak gravitational fields (and for such purposes the Sun’s field is considered weak), light behaves as if there were an index of refraction proportional to the gravitational potential. The stronger the gravitational field, the larger the angu- lar deflection of the light. The Sun is not unique in this regard, and it was quickly appreci- ated that stars in our own galaxy (the MilkyWay) and the combined mass of stars in other galaxies would also, on very rare occasions, produce observable deflections. Variations in the “gravitational” index of refraction would also distort images, stretching them in some directions and shrinking them in others. In the analogous case of terrestrial mirages, the deflections and distortions are due to thermal variations in the index of refraction of air. 36 ) schechter mit physics annual 2003 OTH TERRESTRIAL AND GRAVITATIONAL Bmirages sometimes produce multiple distorted images of the same object. When they do, at least one of the images has the opposite handedness of the object being imaged—it is a mirror image, but distorted. At least one of the other images must have the correct handedness, but it will also be distorted. The French call such distorted images gravitational mirages. In the half century following the confirmation of general relativity, the idea that cosmic mirages might actually be observed was taken seriously byonly a small number of astrophysicists. Most of the papers written on the subject treated them as academic curiosities, far too unlikely to actually be observed.