Periodic Table of the Elements
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Important Role of Dysprosium in Modern Permanent Magnets
The Important Role of Dysprosium in Modern Permanent Magnets Introduction Dysprosium is one of a group of elements called the Rare Earths. Rare earth elements consist of the Lanthanide series of 15 elements plus yttrium and scandium. Yttrium and scandium are included because of similar chemical behavior. The rare earths are divided into light and heavy based on atomic weight and the unique chemical and magnetic properties of each of these categories. Dysprosium (Figure 1) is considered a heavy rare earth element (HREE). One of the more important uses for dysprosium is in neodymium‐iron‐ boron (Neo) permanent magnets to improve the magnets’ resistance to demagnetization, and by extension, its high temperature performance. Neo magnets have become essential for a wide range of consumer, transportation, power generation, defense, aerospace, medical, industrial and other products. Along with terbium (Tb), Dysprosium (Dy) Figure 1: Dysprosium Metal(1) is also used in magnetostrictive devices, but by far the greater usage is in permanent magnets. The demand for Dy has been outstripping its supply. An effect of this continuing shortage is likely to be a slowing of the commercial rollout or a redesigning of a number of Clean Energy applications, including electric traction drives for vehicles and permanent magnet generators for wind turbines. The shortage and associated high prices are also upsetting the market for commercial and industrial motors and products made using them. Background Among the many figures of merit for permanent magnets two are of great importance regarding use of Dy. One key characteristic of a permanent magnet is its resistance to demagnetization, which is quantified by the value of Intrinsic Coercivity (HcJ or Hci). -
Group Vi Elements (The Chalcogens)
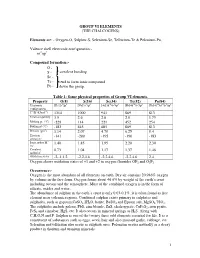
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

1201: Introduction to Aluminium As an Engineering Material
TALAT Lecture 1201 Introduction to Aluminium as an Engineering Material 23 pages, 26 figures (also available as overheads) Basic Level prepared by M H Jacobs * Interdisciplinary Research Centre in Materials The University of Birmingham, UK Objectives To provide an introduction to metallurgical concepts necessary to understand how structural features of aluminium alloys are influenced by alloy composition, processing and heat treatment, and the basic affects of these parameters on the mechanical properties, and hence engineering applications, of the alloys. It is assumed that the reader has some elementary knowledge of physics, chemistry and mathematics. Date of Issue: 1999 EAA - European Aluminium Association 1201 Introduction to Aluminium as an Engineering Material Contents (26 figures) 1201 Introduction to Aluminium as an Engineering Material _____________________ 2 1201.01. Basic mechanical and physical properties__________________________________ 3 1201.01.01 Background _______________________________________________________________ 3 1201.01.02 Commercially pure aluminium ______________________________________________ 4 1201.02 Crystal structure and defects _____________________________________________ 6 1201.02.01 Crystals and atomic bonding __________________________________________________ 6 1201.02.02 Atomic structure of aluminium ______________________________________________ 8 1201.02.03 Crystal structures _________________________________________________________ 8 1201.02.04 Some comments on crystal structures of materials -

NOBELIUM Element Symbol: No Atomic Number: 102
NOBELIUM Element Symbol: No Atomic Number: 102 An initiative of IYC 2011 brought to you by the RACI KERRY LAMB www.raci.org.au NOBELIUM Element symbol: No Atomic number: 102 The credit for discovering Nobelium was disputed with 3 different research teams claiming the discovery. While the first claim dates back to 1957, it was not until 1992 that the International Union of Pure and Applied Chemistry credited the discovery to a research team from Dubna in Russia for work they did in 1966. The element was named Nobelium in 1957 by the first of its claimed discoverers (the Nobel Institute in Sweden). It was named after Alfred Nobel, a Swedish chemist who invented dynamite, held more than 350 patents and bequeathed his fortune to the establishment of the Nobel Prizes. Nobelium is a synthetic element and does not occur in nature and has no known uses other than in scientific research as only tiny amounts of the element have ever been produced. Nobelium is radioactive and most likely metallic. The appearance and properties of Nobelium are unknown as insufficient amounts of the element have been produced. Nobelium is made by the bombardment of curium (Cm) with carbon nuclei. Its most stable isotope, 259No, has a half-life of 58 minutes and decays to Fermium (255Fm) through alpha decay or to Mendelevium (259Md) through electron capture. Provided by the element sponsor Freehills Patent and Trade Mark Attorneys ARTISTS DESCRIPTION I wanted to depict Alfred Nobel, the namesake of Nobelium, as a resolute young man, wearing the Laurel wreath which is the symbol of victory. -

An Alternate Graphical Representation of Periodic Table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt
An Alternate Graphical Representation of Periodic table of Chemical Elements Mohd Abubakr1, Microsoft India (R&D) Pvt. Ltd, Hyderabad, India. [email protected] Abstract Periodic table of chemical elements symbolizes an elegant graphical representation of symmetry at atomic level and provides an overview on arrangement of electrons. It started merely as tabular representation of chemical elements, later got strengthened with quantum mechanical description of atomic structure and recent studies have revealed that periodic table can be formulated using SO(4,2) SU(2) group. IUPAC, the governing body in Chemistry, doesn‟t approve any periodic table as a standard periodic table. The only specific recommendation provided by IUPAC is that the periodic table should follow the 1 to 18 group numbering. In this technical paper, we describe a new graphical representation of periodic table, referred as „Circular form of Periodic table‟. The advantages of circular form of periodic table over other representations are discussed along with a brief discussion on history of periodic tables. 1. Introduction The profoundness of inherent symmetry in nature can be seen at different depths of atomic scales. Periodic table symbolizes one such elegant symmetry existing within the atomic structure of chemical elements. This so called „symmetry‟ within the atomic structures has been widely studied from different prospects and over the last hundreds years more than 700 different graphical representations of Periodic tables have emerged [1]. Each graphical representation of chemical elements attempted to portray certain symmetries in form of columns, rows, spirals, dimensions etc. Out of all the graphical representations, the rectangular form of periodic table (also referred as Long form of periodic table or Modern periodic table) has gained wide acceptance. -

Unit 3 Notes: Periodic Table Notes John Newlands Proposed an Organization System Based on Increasing Atomic Mass in 1864
Unit 3 Notes: Periodic Table Notes John Newlands proposed an organization system based on increasing atomic mass in 1864. He noticed that both the chemical and physical properties repeated every 8 elements and called this the ____Law of Octaves ___________. In 1869 both Lothar Meyer and Dmitri Mendeleev showed a connection between atomic mass and an element’s properties. Mendeleev published first, and is given credit for this. He also noticed a periodic pattern when elements were ordered by increasing ___Atomic Mass _______________________________. By arranging elements in order of increasing atomic mass into columns, Mendeleev created the first Periodic Table. This table also predicted the existence and properties of undiscovered elements. After many new elements were discovered, it appeared that a number of elements were out of order based on their _____Properties_________. In 1913 Henry Mosley discovered that each element contains a unique number of ___Protons________________. By rearranging the elements based on _________Atomic Number___, the problems with the Periodic Table were corrected. This new arrangement creates a periodic repetition of both physical and chemical properties known as the ____Periodic Law___. Periods are the ____Rows_____ Groups/Families are the Columns Valence electrons across a period are There are equal numbers of valence in the same energy level electrons in a group. 1 When elements are arranged in order of increasing _Atomic Number_, there is a periodic repetition of their physical and chemical -

Historical Development of the Periodic Classification of the Chemical Elements
THE HISTORICAL DEVELOPMENT OF THE PERIODIC CLASSIFICATION OF THE CHEMICAL ELEMENTS by RONALD LEE FFISTER B. S., Kansas State University, 1962 A MASTER'S REPORT submitted in partial fulfillment of the requirements for the degree FASTER OF SCIENCE Department of Physical Science KANSAS STATE UNIVERSITY Manhattan, Kansas 196A Approved by: Major PrafeLoor ii |c/ TABLE OF CONTENTS t<y THE PROBLEM AND DEFINITION 0? TEH-IS USED 1 The Problem 1 Statement of the Problem 1 Importance of the Study 1 Definition of Terms Used 2 Atomic Number 2 Atomic Weight 2 Element 2 Periodic Classification 2 Periodic Lav • • 3 BRIEF RtiVJiM OF THE LITERATURE 3 Books .3 Other References. .A BACKGROUND HISTORY A Purpose A Early Attempts at Classification A Early "Elements" A Attempts by Aristotle 6 Other Attempts 7 DOBEREBIER'S TRIADS AND SUBSEQUENT INVESTIGATIONS. 8 The Triad Theory of Dobereiner 10 Investigations by Others. ... .10 Dumas 10 Pettehkofer 10 Odling 11 iii TEE TELLURIC EELIX OF DE CHANCOURTOIS H Development of the Telluric Helix 11 Acceptance of the Helix 12 NEWLANDS' LAW OF THE OCTAVES 12 Newlands' Chemical Background 12 The Law of the Octaves. .........' 13 Acceptance and Significance of Newlands' Work 15 THE CONTRIBUTIONS OF LOTHAR MEYER ' 16 Chemical Background of Meyer 16 Lothar Meyer's Arrangement of the Elements. 17 THE WORK OF MENDELEEV AND ITS CONSEQUENCES 19 Mendeleev's Scientific Background .19 Development of the Periodic Law . .19 Significance of Mendeleev's Table 21 Atomic Weight Corrections. 21 Prediction of Hew Elements . .22 Influence -

Rediscovery of the Elements — a Historical Sketch of the Discoveries
REDISCOVERY OF THE ELEMENTS — A HISTORICAL SKETCH OF THE DISCOVERIES TABLE OF CONTENTS incantations. The ancient Greeks were the first to Introduction ........................1 address the question of what these principles 1. The Ancients .....................3 might be. Water was the obvious basic 2. The Alchemists ...................9 essence, and Aristotle expanded the Greek 3. The Miners ......................14 philosophy to encompass a obscure mixture of 4. Lavoisier and Phlogiston ...........23 four elements — fire, earth, water, and air — 5. Halogens from Salts ...............30 as being responsible for the makeup of all 6. Humphry Davy and the Voltaic Pile ..35 materials of the earth. As late as 1777, scien- 7. Using Davy's Metals ..............41 tific texts embraced these four elements, even 8. Platinum and the Noble Metals ......46 though a over-whelming body of evidence 9. The Periodic Table ................52 pointed out many contradictions. It was taking 10. The Bunsen Burner Shows its Colors 57 thousands of years for mankind to evolve his 11. The Rare Earths .................61 thinking from Principles — which were 12. The Inert Gases .................68 ethereal notions describing the perceptions of 13. The Radioactive Elements .........73 this material world — to Elements — real, 14. Moseley and Atomic Numbers .....81 concrete basic stuff of this universe. 15. The Artificial Elements ...........85 The alchemists, who devoted untold Epilogue ..........................94 grueling hours to transmute metals into gold, Figs. 1-3. Mendeleev's Periodic Tables 95-97 believed that in addition to the four Aristo- Fig. 4. Brauner's 1902 Periodic Table ...98 telian elements, two principles gave rise to all Fig. 5. Periodic Table, 1925 ...........99 natural substances: mercury and sulfur. -

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

JCHS Physical Science 2017-18 Chapter 4
JCHS Physical Science 2017-18 Chapter 4 FSA Science Team Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) www.ck12.org AUTHOR FSA Science Team To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-source, collaborative, and web-based compilation model, CK-12 pioneers and promotes the creation and distribution of high-quality, adaptive online textbooks that can be mixed, modified and printed (i.e., the FlexBook® textbooks). Copyright © 2017 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution-Non-Commercial 3.0 Unported (CC BY-NC 3.0) License (http://creativecommons.org/ licenses/by-nc/3.0/), as amended and updated by Creative Com- mons from time to time (the “CC License”), which is incorporated herein by this reference. -

Hjalmar Fors, the Limits of Matter—Chemistry, Mining & Enlightenment
Miner Econ (2015) 28:131–132 DOI 10.1007/s13563-015-0071-2 BOOK REVIEW Hjalmar Fors, The Limits of Matter—Chemistry, mining & enlightenment The University of Chicago Press Chicago USA 2015 Magnus Ericsson1 Published online: 15 September 2015 # Springer-Verlag Berlin Heidelberg 2015 Swedish chemists have discovered more elements than scien- though at that time not united into one yet), France and tists from any other nation. Over more than 100 years, from England? Part of the answer is surprisingly bureaucratisation the early 18th century to the end of the 19th century, 20 ele- or the creation of an administrative institution called ments of which 18 metals or non-metals and 2 gases, nitrogen Bergskollegium in Swedish or English Bureau of Mines as and chlorine were independently isolated and described. Some Hjalmar Fors calls it in his new and path-breaking study of these are as follows: The Limits of Matter—Chemistry, mining & enlightenment. Another part is the need of improved processes and better Element Name Year of discovery yields in Swedish mines, which played such a vital role in Cobalt Brandt 1735 the Swedish economy during this period. The Swedish ruling Nickel Cronstedt 1751 groups saw these demands and founded the Bureau of Mines, Manganese Gahn 1774 which acted in several ways to solve problems, that had arisen in the mining industry. R&D in those days was highly Molybdenum Hjelm 1781 applied but nevertheless led to important purely scientific Yttrium Gadolin 1794 results. Tantalum Ekeberg 1802 The Bureau was set up in 1634 based on a predecessor, Cerium Berzelius 1803 which was started already in 1630. -

Actinide Ground-State Properties-Theoretical Predictions
Actinide Ground-State Properties Theoretical predictions John M. Wills and Olle Eriksson electron-electron correlations—the electronic energy of the ground state of or nearly fifty years, the actinides interactions among the 5f electrons and solids, molecules, and atoms as a func- defied the efforts of solid-state between them and other electrons—are tional of electron density. The DFT Ftheorists to understand their expected to affect the bonding. prescription has had such a profound properties. These metals are among Low-symmetry crystal structures, impact on basic research in both the most complex of the long-lived relativistic effects, and electron- chemistry and solid-state physics that elements, and in the solid state, they electron correlations are very difficult Walter Kohn, its main inventor, was display some of the most unusual to treat in traditional electronic- one of the recipients of the 1998 behaviors of any series in the periodic structure calculations of metals and, Nobel Prize in Chemistry. table. Very low melting temperatures, until the last decade, were outside the In general, it is not possible to apply large anisotropic thermal-expansion realm of computational ability. And DFT without some approximation. coefficients, very low symmetry crystal yet, it is essential to treat these effects But many man-years of intense research structures, many solid-to-solid phase properly in order to understand the have yielded reliable approximate transitions—the list is daunting. Where physics of the actinides. Electron- expressions for the total energy in does one begin to put together an electron correlations are important in which all terms, except for a single- understanding of these elements? determining the degree to which 5f particle kinetic-energy term, can be In the last 10 years, together with electrons are localized at lattice sites.