Neutron Beta-Decay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
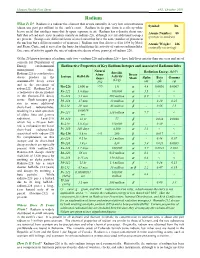
Radium What Is It? Radium Is a Radioactive Element That Occurs Naturally in Very Low Concentrations Symbol: Ra (About One Part Per Trillion) in the Earth’S Crust
Human Health Fact Sheet ANL, October 2001 Radium What Is It? Radium is a radioactive element that occurs naturally in very low concentrations Symbol: Ra (about one part per trillion) in the earth’s crust. Radium in its pure form is a silvery-white heavy metal that oxidizes immediately upon exposure to air. Radium has a density about one- Atomic Number: 88 half that of lead and exists in nature mainly as radium-226, although several additional isotopes (protons in nucleus) are present. (Isotopes are different forms of an element that have the same number of protons in the nucleus but a different number of neutrons.) Radium was first discovered in 1898 by Marie Atomic Weight: 226 and Pierre Curie, and it served as the basis for identifying the activity of various radionuclides. (naturally occurring) One curie of activity equals the rate of radioactive decay of one gram (g) of radium-226. Of the 25 known isotopes of radium, only two – radium-226 and radium-228 – have half-lives greater than one year and are of concern for Department of Energy environmental Radioactive Properties of Key Radium Isotopes and Associated Radionuclides management sites. Natural Specific Radiation Energy (MeV) Radium-226 is a radioactive Abun- Decay Isotope Half-Life Activity decay product in the dance Mode Alpha Beta Gamma (Ci/g) uranium-238 decay series (%) (α) (β) (γ) and is the precursor of Ra-226 1,600 yr >99 1.0 α 4.8 0.0036 0.0067 radon-222. Radium-228 is a radioactive decay product Rn-222 3.8 days 160,000 α 5.5 < < in the thorium-232 decay Po-218 3.1 min 290 million α 6.0 < < series. -

Neutrinoless Double Beta Decay
REPORT TO THE NUCLEAR SCIENCE ADVISORY COMMITTEE Neutrinoless Double Beta Decay NOVEMBER 18, 2015 NLDBD Report November 18, 2015 EXECUTIVE SUMMARY In March 2015, DOE and NSF charged NSAC Subcommittee on neutrinoless double beta decay (NLDBD) to provide additional guidance related to the development of next generation experimentation for this field. The new charge (Appendix A) requests a status report on the existing efforts in this subfield, along with an assessment of the necessary R&D required for each candidate technology before a future downselect. The Subcommittee membership was augmented to replace several members who were not able to continue in this phase (the present Subcommittee membership is attached as Appendix B). The Subcommittee solicited additional written input from the present worldwide collaborative efforts on double beta decay projects in order to collect the information necessary to address the new charge. An open meeting was held where these collaborations were invited to present material related to their current projects, conceptual designs for next generation experiments, and the critical R&D required before a potential down-select. We also heard presentations related to nuclear theory and the impact of future cosmological data on the subject of NLDBD. The Subcommittee presented its principal findings and comments in response to the March 2015 charge at the NSAC meeting in October 2015. The March 2015 charge requested the Subcommittee to: Assess the status of ongoing R&D for NLDBD candidate technology demonstrations for a possible future ton-scale NLDBD experiment. For each candidate technology demonstration, identify the major remaining R&D tasks needed ONLY to demonstrate downselect criteria, including the sensitivity goals, outlined in the NSAC report of May 2014. -

RIB Production by Photofission in the Framework of the ALTO Project
Available online at www.sciencedirect.com NIM B Beam Interactions with Materials & Atoms Nuclear Instruments and Methods in Physics Research B 266 (2008) 4092–4096 www.elsevier.com/locate/nimb RIB production by photofission in the framework of the ALTO project: First experimental measurements and Monte-Carlo simulations M. Cheikh Mhamed *, S. Essabaa, C. Lau, M. Lebois, B. Roussie`re, M. Ducourtieux, S. Franchoo, D. Guillemaud Mueller, F. Ibrahim, J.F. LeDu, J. Lesrel, A.C. Mueller, M. Raynaud, A. Said, D. Verney, S. Wurth Institut de Physique Nucle´aire, IN2P3-CNRS/Universite´ Paris-Sud, F-91406 Orsay Cedex, France Available online 11 June 2008 Abstract The ALTO facility (Acce´le´rateur Line´aire aupre`s du Tandem d’Orsay) has been built and is now under commissioning. The facility is intended for the production of low energy neutron-rich ion-beams by ISOL technique. This will open new perspectives in the study of nuclei very far from the valley of stability. Neutron-rich nuclei are produced by photofission in a thick uranium carbide target (UCx) using a 10 lA, 50 MeV electron beam. The target is the same as that already had been used on the previous deuteron based fission ISOL setup (PARRNE [F. Clapier et al., Phys. Rev. ST-AB (1998) 013501.]). The intended nominal fission rate is about 1011 fissions/s. We have studied the adequacy of a thick carbide uranium target to produce neutron-rich nuclei by photofission by means of Monte-Carlo simulations. We present the production rates in the target and after extraction and mass separation steps. -

The Use of Cosmic-Rays in Detecting Illicit Nuclear Materials
The use of Cosmic-Rays in Detecting Illicit Nuclear Materials Timothy Benjamin Blackwell Department of Physics and Astronomy University of Sheffield This dissertation is submitted for the degree of Doctor of Philosophy 05/05/2015 Declaration I hereby declare that except where specific reference is made to the work of others, the contents of this dissertation are original and have not been submitted in whole or in part for consideration for any other degree or qualification in this, or any other Univer- sity. This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. Timothy Benjamin Blackwell 05/05/2015 Acknowledgements This thesis could not have been completed without the tremendous support of many people. Firstly I would like to express special appreciation and thanks to my academic supervisor, Dr Vitaly A. Kudryavtsev for his expertise, understanding and encourage- ment. I have enjoyed our many discussions concerning my research topic throughout the PhD journey. I would also like to thank my viva examiners, Professor Lee Thomp- son and Dr Chris Steer, for the time you have taken out of your schedules, so that I may take this next step in my career. Thanks must also be given to Professor Francis Liven, Professor Neil Hyatt and the rest of the Nuclear FiRST DTC team, for the initial oppor- tunity to pursue postgraduate research. Appreciation is also given to the University of Sheffield, the HEP group and EPRSC for providing me with the facilities and funding, during this work. -

Chapter 3 the Fundamentals of Nuclear Physics Outline Natural
Outline Chapter 3 The Fundamentals of Nuclear • Terms: activity, half life, average life • Nuclear disintegration schemes Physics • Parent-daughter relationships Radiation Dosimetry I • Activation of isotopes Text: H.E Johns and J.R. Cunningham, The physics of radiology, 4th ed. http://www.utoledo.edu/med/depts/radther Natural radioactivity Activity • Activity – number of disintegrations per unit time; • Particles inside a nucleus are in constant motion; directly proportional to the number of atoms can escape if acquire enough energy present • Most lighter atoms with Z<82 (lead) have at least N Average one stable isotope t / ta A N N0e lifetime • All atoms with Z > 82 are radioactive and t disintegrate until a stable isotope is formed ta= 1.44 th • Artificial radioactivity: nucleus can be made A N e0.693t / th A 2t / th unstable upon bombardment with neutrons, high 0 0 Half-life energy protons, etc. • Units: Bq = 1/s, Ci=3.7x 1010 Bq Activity Activity Emitted radiation 1 Example 1 Example 1A • A prostate implant has a half-life of 17 days. • A prostate implant has a half-life of 17 days. If the What percent of the dose is delivered in the first initial dose rate is 10cGy/h, what is the total dose day? N N delivered? t /th t 2 or e Dtotal D0tavg N0 N0 A. 0.5 A. 9 0.693t 0.693t B. 2 t /th 1/17 t 2 2 0.96 B. 29 D D e th dt D h e th C. 4 total 0 0 0.693 0.693t /th 0.6931/17 C. -

Radioactive Decay
North Berwick High School Department of Physics Higher Physics Unit 2 Particles and Waves Section 3 Fission and Fusion Section 3 Fission and Fusion Note Making Make a dictionary with the meanings of any new words. Einstein and nuclear energy 1. Write down Einstein’s famous equation along with units. 2. Explain the importance of this equation and its relevance to nuclear power. A basic model of the atom 1. Copy the components of the atom diagram and state the meanings of A and Z. 2. Copy the table on page 5 and state the difference between elements and isotopes. Radioactive decay 1. Explain what is meant by radioactive decay and copy the summary table for the three types of nuclear radiation. 2. Describe an alpha particle, including the reason for its short range and copy the panel showing Plutonium decay. 3. Describe a beta particle, including its range and copy the panel showing Tritium decay. 4. Describe a gamma ray, including its range. Fission: spontaneous decay and nuclear bombardment 1. Describe the differences between the two methods of decay and copy the equation on page 10. Nuclear fission and E = mc2 1. Explain what is meant by the terms ‘mass difference’ and ‘chain reaction’. 2. Copy the example showing the energy released during a fission reaction. 3. Briefly describe controlled fission in a nuclear reactor. Nuclear fusion: energy of the future? 1. Explain why nuclear fusion might be a preferred source of energy in the future. 2. Describe some of the difficulties associated with maintaining a controlled fusion reaction. -

Two-Proton Radioactivity 2
Two-proton radioactivity Bertram Blank ‡ and Marek P loszajczak † ‡ Centre d’Etudes Nucl´eaires de Bordeaux-Gradignan - Universit´eBordeaux I - CNRS/IN2P3, Chemin du Solarium, B.P. 120, 33175 Gradignan Cedex, France † Grand Acc´el´erateur National d’Ions Lourds (GANIL), CEA/DSM-CNRS/IN2P3, BP 55027, 14076 Caen Cedex 05, France Abstract. In the first part of this review, experimental results which lead to the discovery of two-proton radioactivity are examined. Beyond two-proton emission from nuclear ground states, we also discuss experimental studies of two-proton emission from excited states populated either by nuclear β decay or by inelastic reactions. In the second part, we review the modern theory of two-proton radioactivity. An outlook to future experimental studies and theoretical developments will conclude this review. PACS numbers: 23.50.+z, 21.10.Tg, 21.60.-n, 24.10.-i Submitted to: Rep. Prog. Phys. Version: 17 December 2013 arXiv:0709.3797v2 [nucl-ex] 23 Apr 2008 Two-proton radioactivity 2 1. Introduction Atomic nuclei are made of two distinct particles, the protons and the neutrons. These nucleons constitute more than 99.95% of the mass of an atom. In order to form a stable atomic nucleus, a subtle equilibrium between the number of protons and neutrons has to be respected. This condition is fulfilled for 259 different combinations of protons and neutrons. These nuclei can be found on Earth. In addition, 26 nuclei form a quasi stable configuration, i.e. they decay with a half-life comparable or longer than the age of the Earth and are therefore still present on Earth. -

Heavy Element Nucleosynthesis
Heavy Element Nucleosynthesis A summary of the nucleosynthesis of light elements is as follows 4He Hydrogen burning 3He Incomplete PP chain (H burning) 2H, Li, Be, B Non-thermal processes (spallation) 14N, 13C, 15N, 17O CNO processing 12C, 16O Helium burning 18O, 22Ne α captures on 14N (He burning) 20Ne, Na, Mg, Al, 28Si Partly from carbon burning Mg, Al, Si, P, S Partly from oxygen burning Ar, Ca, Ti, Cr, Fe, Ni Partly from silicon burning Isotopes heavier than iron (as well as some intermediate weight iso- topes) are made through neutron captures. Recall that the prob- ability for a non-resonant reaction contained two components: an exponential reflective of the quantum tunneling needed to overcome electrostatic repulsion, and an inverse energy dependence arising from the de Broglie wavelength of the particles. For neutron cap- tures, there is no electrostatic repulsion, and, in complex nuclei, virtually all particle encounters involve resonances. As a result, neutron capture cross-sections are large, and are very nearly inde- pendent of energy. To appreciate how heavy elements can be built up, we must first consider the lifetime of an isotope against neutron capture. If the cross-section for neutron capture is independent of energy, then the lifetime of the species will be ( )1=2 1 1 1 µn τn = ≈ = Nnhσvi NnhσivT Nnhσi 2kT For a typical neutron cross-section of hσi ∼ 10−25 cm2 and a tem- 8 9 perature of 5 × 10 K, τn ∼ 10 =Nn years. Next consider the stability of a neutron rich isotope. If the ratio of of neutrons to protons in an atomic nucleus becomes too large, the nucleus becomes unstable to beta-decay, and a neutron is changed into a proton via − (Z; A+1) −! (Z+1;A+1) + e +ν ¯e (27:1) The timescale for this decay is typically on the order of hours, or ∼ 10−3 years (with a factor of ∼ 103 scatter). -

2.3 Neutrino-Less Double Electron Capture - Potential Tool to Determine the Majorana Neutrino Mass by Z.Sujkowski, S Wycech
DEPARTMENT OF NUCLEAR SPECTROSCOPY AND TECHNIQUE 39 The above conservatively large systematic hypothesis. TIle quoted uncertainties will be soon uncertainty reflects the fact that we did not finish reduced as our analysis progresses. evaluating the corrections fully in the current analysis We are simultaneously recording a large set of at the time of this writing, a situation that will soon radiative decay events for the processes t e'v y change. This result is to be compared with 1he and pi-+eN v y. The former will be used to extract previous most accurate measurement of McFarlane the ratio FA/Fv of the axial and vector form factors, a et al. (Phys. Rev. D 1984): quantity of great and longstanding interest to low BR = (1.026 ± 0.039)'1 I 0 energy effective QCD theory. Both processes are as well as with the Standard Model (SM) furthermore very sensitive to non- (V-A) admixtures in prediction (Particle Data Group - PDG 2000): the electroweak lagLangian, and thus can reveal BR = (I 038 - 1.041 )*1 0-s (90%C.L.) information on physics beyond the SM. We are currently analyzing these data and expect results soon. (1.005 - 1.008)* 1W') - excl. rad. corr. Tale 1 We see that even working result strongly confirms Current P1IBETA event sxpelilnentstatistics, compared with the the validity of the radiative corrections. Another world data set. interesting comparison is with the prediction based on Decay PIBETA World data set the most accurate evaluation of the CKM matrix n >60k 1.77k element V d based on the CVC hypothesis and ihce >60 1.77_ _ _ results -

Double-Beta Decay from First Principles
Double-Beta Decay from First Principles J. Engel April 23, 2020 Goal is set of matrix elements with real error bars by May, 2021 DBD Topical Theory Collaboration Lattice QCD Data Chiral EFT Similarity Renormalization Group Ab-Initio Many-Body Methods Harmonic No-Core Quantum Oscillator Basis Shell Model Monte Carlo Effective Theory Light Nuclei (benchmarking) DFT-Inspired Coupled Multi-reference In-Medium SRG Clusters In-Medium SRG for Shell Model Heavy Nuclei DFT Statistical Model Averaging (for EDMs) Shell Model Goal is set of matrix elements with real error bars by May, 2021 DBD Topical Theory Collaboration Haxton HOBET Walker-Loud McIlvain LQCD Brantley Monge- Johnson Camacho Ramsey- SM Musolf Horoi Engel EFT SM Cirigliano Nicholson Mereghetti "DFT" EFT LQCD QMC Carlson Jiao Quaglioni Vary SRG NC-SM Yao Papenbrock LQCD = Latice QCD Hagen EFT = Effective Field Theory Bogner QMC = Quantum Monte Carlo Morris Hergert DFT = Densty Functional Theory Sun Nazarewicz Novario SRG = Similarity Renormilazation Group More IM-SRG IM-SRG = In-Medium SRG Coupled Clusters DFT HOBET = Harmonic-Oscillator-Based Statistics Effective Theory NC-SM = No-Core Shell Model SM = Shell Model DBD Topical Theory Collaboration Haxton HOBET Walker-Loud McIlvain LQCD Brantley Monge- Johnson Camacho Ramsey- SM Musolf Horoi Engel EFT SM Cirigliano Nicholson Mereghetti "DFT" EFT LQCD QMC Carlson Jiao Quaglioni Vary Goal is set of matrixSRG elementsNC-SM with Yao real error bars by May, 2021 Papenbrock LQCD = Latice QCD Hagen EFT = Effective Field Theory Bogner QMC = Quantum Monte Carlo Morris Hergert DFT = Densty Functional Theory Sun Nazarewicz Novario SRG = Similarity Renormilazation Group More IM-SRG IM-SRG = In-Medium SRG Coupled Clusters DFT HOBET = Harmonic-Oscillator-Based Statistics Effective Theory NC-SM = No-Core Shell Model SM = Shell Model Part 0 0νββ Decay and neutrinos are their own antiparticles.. -

"Indoor Radon and Radon Decay Product Measurement Device Protocols"
"Indoor Radon and Radon Decay Product Measurement Device Protocols" U.S. Environmental Protection Agency Office of Air and Radiation (6604J) EPA 402-R-92-004, July 1992 (revised) Table of Contents Disclaimer Acknowledgements Significant Changes in This Revision Radon and Radon Decay Product Measurement Methods Section 1: General Considerations 1.1 Introduction and Background 1.2 General Guidance on Measurement Strategy, Measurement Conditions, Device Location Selection, and Documentation 1.3 Quality Assurance Section 2: Indoor Radon Measurement Device Protocols 2.1 Protocol for Using Continuous Radon Monitors (CR) to Measure Indoor Radon Concentrations 2.2 Protocol for Using Alpha Track Detectors (AT or ATD) to Measure Indoor Radon Concentrations 2.4 Protocol for Using Activated Charcoal Adsorption Devices (AC) to Measure Indoor Radon Concentrations 2.5 Protocol for Using Charcoal Liquid Scintillation (LS) Devices to Measure Indoor Radon Concentrations 2.6 Protocol for Using Grab Radon Sampling (GB, GC, GS), Pump/Collapsible Bag Devices (PB), and Three-Day Integrating Evacuated Scintillation Cells (SC) to Measure Indoor Radon Concentrations 2.7 Interim Protocol for Using Unfiltered Track Detectors (UT) to Measure Indoor Radon Concentrations Section 3: Indoor Radon Decay Product Measurement Device Protocols 3.1 Protocol for Using Continuous Working Level Monitors (CW) to Measure Indoor Radon Decay Product Concentrations 3.2 Protocol for Using Radon Progeny Integrating Sampling Units (RPISU or RP) to Measure Indoor Radon Decay Product Concentrations 3.3 Protocol for Using Grab Sampling-Working Level (GW) to Measure Indoor Radon Decay Product Concentrations Glossary References Please Note: EPA closed its National Radon Proficiency Program (RPP) in 1998. -

Energy What Is a Nuclear Reaction?
4/16/2016 Option C: Energy C.3 : Nuclear Fusion and Fission What is a nuclear reaction? • A nuclear reaction is any reaction that involves the nucleus. • These reactions change the identity of an atom, as opposed to chemical reactions which only involve valence electrons. 1 4/16/2016 The nucleus • The nucleus is made up of protons and neutrons. • We know what the protons do – they provide an electrostatic attraction to the electrons close… but what about the neutrons? The Neutrons • The major function of the neutrons is to hold the nucleus together. • The neutrons provide a strong nuclear force of attraction within the nucleus, counteracting the repulsion between the positively charged protons. 2 4/16/2016 How is the nucleus held together? • In the 1930’s it was first observed that the mass of an atoms nucleus is less than the sum of the masses of the protons + neutrons…? • Some of the mass of the nucleus is converted into energy to hold the nucleus together. 3 4/16/2016 Mass Defect • The difference in mass of the nucleus and it’s parts is referred to as the mass defect, and the energy (e=mc 2) it provided is called the nuclear binding energy. 4 4/16/2016 Nuclear vs. Chemical Reactions • This nuclear binding energy is released during nuclear reactions (fission & fusion), and is ~1,000,000X greater than the chemical bond energy released during chemical reactions. What makes an isotope radioactive? • Elements are radioactive when their nucleus is unstable. • The stabilizing force of the neutrons is effective for smaller elements, though all elements above lead are radioactive.