Redox Reaction & Equivalent Concept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics of Ion Exchange
Chapter 1 Thermodynamics of Ion Exchange Ayben Kilislioğlu Additional information is available at the end of the chapter http://dx.doi.org/10.5772/53558 1. Introduction 1.1. Ion exchange equilibria During an ion exchange process, ions are essentially stepped from the solvent phase to the solid surface. As the binding of an ion takes place at the solid surface, the rotational and translational freedom of the solute are reduced. Therefore, the entropy change (ΔS) during ion exchange is negative. For ion exchange to be convenient, Gibbs free energy change (ΔG) must be negative, which in turn requires the enthalpy change to be negative because ΔG = ΔH - TΔS. Both enthalpic (ΔHo) and entropic (ΔSo) changes help decide the overall selectivity of the ion-exchange process [Marcus Y., SenGupta A. K. 2004]. Thermodynamics have great efficiency on the impulsion of ion exchange. It also sets the equilibrium distribution of ions between the solution and the solid. A discussion about the role of thermodynamics relevant to both of these phenomena was done by researchers [Araujo R., 2004]. As the basic rule of ion exchange, one type of a free mobile ion of a solution become fixed on the solid surface by releasing a different kind of an ion from the solid surface. It is a reversible process which means that there is no permanent change on the solid surface by the process. Ion exchange has many applications in different fields like enviromental, medical, technological,.. etc. To evaluate the properties and efficiency of the ion exchange one must determine the equilibrium conditions. -

Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL
United States Office of Pollution EPA 744-B-97-001 Environmental Protection Prevention and Toxics June 1997 Agency (7406) Polymer Exemption Guidance Manual POLYMER EXEMPTION GUIDANCE MANUAL 5/22/97 A technical manual to accompany, but not supersede the "Premanufacture Notification Exemptions; Revisions of Exemptions for Polymers; Final Rule" found at 40 CFR Part 723, (60) FR 16316-16336, published Wednesday, March 29, 1995 Environmental Protection Agency Office of Pollution Prevention and Toxics 401 M St., SW., Washington, DC 20460-0001 Copies of this document are available through the TSCA Assistance Information Service at (202) 554-1404 or by faxing requests to (202) 554-5603. TABLE OF CONTENTS LIST OF EQUATIONS............................ ii LIST OF FIGURES............................. ii LIST OF TABLES ............................. ii 1. INTRODUCTION ............................ 1 2. HISTORY............................... 2 3. DEFINITIONS............................. 3 4. ELIGIBILITY REQUIREMENTS ...................... 4 4.1. MEETING THE DEFINITION OF A POLYMER AT 40 CFR §723.250(b)... 5 4.2. SUBSTANCES EXCLUDED FROM THE EXEMPTION AT 40 CFR §723.250(d) . 7 4.2.1. EXCLUSIONS FOR CATIONIC AND POTENTIALLY CATIONIC POLYMERS ....................... 8 4.2.1.1. CATIONIC POLYMERS NOT EXCLUDED FROM EXEMPTION 8 4.2.2. EXCLUSIONS FOR ELEMENTAL CRITERIA........... 9 4.2.3. EXCLUSIONS FOR DEGRADABLE OR UNSTABLE POLYMERS .... 9 4.2.4. EXCLUSIONS BY REACTANTS................ 9 4.2.5. EXCLUSIONS FOR WATER-ABSORBING POLYMERS........ 10 4.3. CATEGORIES WHICH ARE NO LONGER EXCLUDED FROM EXEMPTION .... 10 4.4. MEETING EXEMPTION CRITERIA AT 40 CFR §723.250(e) ....... 10 4.4.1. THE (e)(1) EXEMPTION CRITERIA............. 10 4.4.1.1. LOW-CONCERN FUNCTIONAL GROUPS AND THE (e)(1) EXEMPTION................. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -
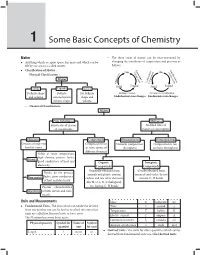
1 Some Basic Concepts of Chemistry
Some Basic Concepts of Chemistry 1 1 Some Basic Concepts of Chemistry Matter – The three states of matter can be inter-converted by Anything which occupies space, has mass and which can be changing the conditions of temperature and pressure as felt by our senses is called matter. follows : Ev Liq Classification of Matter : Co GasGVa ap as uefacti n po orat tion ndensa – tion Physical Classification : io riza at io on n o tion tion Matter lim ndensa or r Deposi Co Sub or Solid Liquid Solid Liquid Solids Liquids Gases Definite shape Definite No definite Melting or Fusion Freezing or Crystallization and volume volume but no shape and Endothermic state changes Exothermic state changes definite shape volume – Chemical Classification : Matter Pure Substances Mixtures Fixed ratio of masses No fixed ratio of of constituents masses of constituents Elements Compounds Homogeneous Heterogeneous Consists of only one Composed of two Uniform composition Composition is not kind of atoms or more atoms of throughout uniform throughout different elements Solids at room temperature, high density, possess lustre, Metals good conductors of heat and Organic Inorganic electricity Compounds Compounds Originally obtained from Usually obtained from Brittle, do not possess animals and plants, contain minerals and rocks, do not lustre, poor conductors Non-metals carbon and few other elements contain C–H bonds of heat and electricity like H, O, S, N, X (halogens), Possess characteristics etc. having C–H bonds Metalloids of both metals and non- metals Units and Measurements Mass m kilogram kg Fundamental Units : The units which can neither be derived Time t second s from one another nor can be further resolved into any other Temperature T kelvin K units are called fundamental units or basic units. -

Basic Concepts and Laws of Chemistry. Guidelines and Objectives for Self-Study Courses for Students in All Specialties / O.Y
THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» O.Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro 2016 THE MINISTRY OF EDUCATION AND SCIENCE OF UKRAINE STATE HIGHER EDUCATIONAL INSTITUTION «NATIONAL MINING UNIVERSITY» FACULTY OF GEOLOGICAL PROSPECTING Departament of Chemestry O. Y. Svietkina, O.B Netyaga, G.V Tarasova BASIC CONCEPTS AND LAWS OF CHEMISTRY. Guidelines and objectives for self-study courses for students in all specialties Dnipro NMU 2016 Svietkina O. Y. Basic concepts and laws of chemistry. Guidelines and objectives for self-study courses for students in all specialties / O.Y. Svietkina, O.B. Netyaga, G.V. Tarasova; Ministry of eduk. and sien of Ukrain, Nation. min. univer. – D . : NMU, 2016. – 20 p. Автори: О.Ю. Свєткіна, проф., д-р техн. наук (передмова, розділ 2); О.Б. Нетяга, старш. викл. (розділ 1); Г.В. Тарасова, асист. (розділ 2). Затверджено методичною комісією з галузі знань 10. Природничі науки за поданням кафедри хімії (протокол № 3 від 08.11.2016). The theoretical themes of "Basic concepts and laws of chemistry", are examples of solving common tasks, in order to consolidate the material there are presented self-study tasks for solving. Розглянуто теоретичні положення теми «Основні поняття й закони хімії», наведено приклади розв’язку типових задач з метою закріплення матеріалу, подано задачі для самостійного розв’язування. Відповідальна за випуск завідувач кафедри хімії, д-р техн. наук, проф. О.Ю. Свєткіна. Introduction Chemistry studies such form of substance motion which assumes qualitative change of matters i.e. -

(I) Determination of the Equivalent Weight and Pka of an Organic Acid
Experiment 4 (i) Determination of the Equivalent Weight and pKa of an Organic Acid Discussion This experiment is an example of a common research procedure. Chemists often use two or more analytical techniques to study the same system. These experiments can give complementary qualitative and quantitative information concerning an unknown substance. I. Titration of Acids and Bases in Aqueous Solutions The almost instantaneous reaction between acids and bases in aqueous solution produce changes in pH which one can monitor. Two techniques are useful for detecting the equivalence point: (1) colorimetry, using an acid-base color indicator - a dye which undergoes a sharp change in color in a region of pH covering the equivalence point and (2) potentiometry, using a potentiometer (pH meter) to record the sharp change at the equivalence point in the potential difference between an electrode (usually a glass electrode) and the solution whose pH is undergoing change as a result of the addition of acid or base. For example, in the case of the titration of a weak monoprotic acid HA using sodium hydroxide solution we may write: + - NaOH + HA → Na + A + H2O (4.1) Applying the law of mass action to the ionization equilibrium for the weak acid in water: + - HA + H2O H3O + A (4.2) we may write (in dilute solutions [H2O] is essentially constant) [H O+ ][A − ] 3 = K (4.3) [HA] a where Ka is the acid ionization constant (constant at any given temperature). This expression is valid for + - all aqueous solutions containing hydronium ions (H3O ), A ions, and the un-ionized molecules HA. -

PHARMACY CALCULATIONS •Explain Importance of Calculations in Determining Days Supply •Describe Consequences of Calculation Errors
7/7/2016 THE OBJECTIVES INCLUDE THE FOLLOWING: •Discuss importance of calculations in a pharmacy practice. •Outline steps to avoid calculation errors 2016 ANNUAL MEETING •Explain importance of reading the prescription carefully then performing calculations PHARMACY CALCULATIONS •Explain importance of calculations in determining days supply •Describe consequences of calculation errors. •Perform calculations during the presentation 2016 ANNUAL MEETING 4 ADDITIONAL LEARNING OBJECTIVES •Understand how the concentration of electrolyte 2016 ANNUAL MEETING solutions are expressed and calculations of m.Eq. •Understand the importance of iso-tonicity in SUNIL S. JAMBHEKAR, B. PHARM.; M.S., PH.D ophthalmic solutions. PROFESSOR, PHARMACEUTICAL SCIENCES LECOM BRADENTON, SCHOOL OF PHARMACY •Perform calculations, using a prescription, to BRADENTON, FL34202 determine the quantity of a liquid product required [email protected] to fill the prescription accurately. 2016 ANNUAL MEETING 5 ADDITIONAL LEARNING OBJECTIVES •Perform calculations, using a prescription, to 2016 ANNUAL MEETING determine the # of day supply for a specific DISCLOSURE product. I DO NOT HAVE A VESTED INTEREST IN OR AFFILIATION •Perform calculations to determine m.Eq WITH ANY CORPORATE ORGANIZATION OFFERING FINANCIAL SUPPORT OR GRANT MONIES FOR THIS CONTINUING strength of an electrolyte solution. EDUCATION ACTIVITY, OR ANY AFFILIATION WITH AN ORGANIZATION WHOSE PHILOSOPHY COULD POTENTIALLY •Perform calculations to prepare an isotonic BIAS MY PRESENTATION ophthalmic solution. 2016 ANNUAL MEETING 6 1 7/7/2016 IMPORTANCE OF CALCULATIONS IN THE PHARMACY PRACTICE: •It is consistent with the practice and philosophy of pharmaceutical care. •Insurance companies will reimburse you or •Patient safety is of utmost importance. your employer only for the quantity written on the prescription and not for the quantity •Your pharmacy is the last check point for the dispensed. -

WO 2019/055177 Al 21 March 2019 (21.03.2019) W 1P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization I International Bureau (10) International Publication Number (43) International Publication Date WO 2019/055177 Al 21 March 2019 (21.03.2019) W 1P O PCT (51) International Patent Classification: Declarations under Rule 4.17: C08G 65/02 (2006.01) C08G 18/32 (2006.01) — as to applicant's entitlement to apply for and be granted a C08G 65/26 (2006.01) patent (Rule 4.17(H)) (21) International Application Number: — as to the applicant's entitlement to claim the priority of the PCT/US20 18/047252 earlier application (Rule 4.17(Hi)) (22) International Filing Date: Published: 2 1 August 2018 (21.08.2018) — with international search report (Art. 21(3)) (25) Filing Language: English (26) Publication Language: English (30) Priority Data: 62/559,109 15 September 2017 (15.09.2017) US (71) Applicant: DOW GLOBAL TECHNOLOGIES LLC [US/US]; 2040 Dow Center, Midland, Michigan 48674 (US). (72) Inventors: MASY, Jean-Paul; Herbert H . Dowweg 5, 4542 NM Hoek, Postbus 48, 4530AA Terneuzen (NL). BABB, David A.; 230 1N . Brazosport Boulevard, Freeport, Texas 77541 (US). (74) Agent: Gary C Cohn PLLC; 325 7th Avenue, #203, San Diego, California 92101 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available) : AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -
Some Basic Concepts of Chemistry
Chapter 1 Some Basic Concepts of Chemistry Solutions SECTION - A Objective Type Questions (One option is correct) 1. The total number of ions present in 1 ml of 0.1 M barium nitrate solution is (1) 6.02 × 108 (2) 6.02 × 1010 (3) 3.0 × 6.02 × 1019 (4) 3.0 × 6.02 × 108 Sol. Answer (3) Number of molecules of Ba(NO3)2 1 0.1 N = 1000 A –4 = 10 NA ∵ 1 molecule Ba(NO3)2 gives 3 ions –4 –4 10 NA molecules will give = 10 NA × 3 = 3 × 6.02 × 1019 = 1.806 × 1020 2. Which of the following have lowest weight? (1) 6.023 × 1022 molecules of glucose (2) 18 ml of water at 4°C (3) 11200 ml of CH4 at STP (4) 5.6 litre of CO2 at STP Sol. Answer (3) 11200 ml of CH4 at STP has lowest weight i.e., 8 g. 3. The largest number of molecules are in (1) 28 g of CO (2) 46 g of C2H5OH (3) 36 g of H2O (4) 54 g of N2O5 Sol. Answer (3) Sample Number of molecules 28 NA 28 g CO 28 = NA 46 NA 46 g C2H5OH 46 = NA 36 NA 36 g of H2O 18 = 2NA 54 NA NA 54 g N2O5 108 2 36 g H2O contains maximum number of molecules 2 Some Basic Concepts of Chemistry Solution of Assignment 4. The volume of 0.1 M Ca(OH)2 needed for the neutralization of 40 ml of 0.05 M oxalic acid is (1) 10 ml (2) 20 ml (3) 30 ml (4) 40 ml Sol. -

Determination of the Equivalent Weight and the Ka Or Kb for a Weak Acid Or Base INTRODUCTION Chemists Frequently Make Use of the Equivalent Weight (Eq
Determination of the Equivalent Weight and the Ka or Kb for a Weak Acid or Base INTRODUCTION Chemists frequently make use of the equivalent weight (eq. wt.) as the basis for volumetric calculations. The meaning of equivalent weight can change depending upon the type of reaction which serves as the basis for analysis, i.e., neutralization, oxidation-reduction, precipitation, or complex formation. Furthermore, it should be noted that the chemical behavior of a substance must be carefully specified if its equivalent weight is to be unambiguously defined. The equivalent weight of a substance involved in a neutralization (acid/base) reaction is defined as that weight which reacts with or contributes 1 gram formula weight of hydrogen ion in that reaction. This is discussed at some length in your textbook. In this experiment, you will be given a compound which is either a pure weak acid or a pure weak base. You are asked to determine the nature of the substance (i.e. is it an acid or a base?), its equivalent weight, and its dissociation constant. If possible, using the values you obtain and the CRC Handbook, identify your compound. REAGENTS Ethanol, phenolphthalein and methyl red indicators are provided for you. PROCEDURE 1. Obtain your unknown weak acid or base, and dry it as instructed to do so if it is a solid. 2. To determine the nature of your compound: Dissolve a small amount of your unknown in water. If you find your unknown to be rather insoluble in water, try heating the solution. If this also proves unsuccessful, you will need to switch to a 10% ethanol/water mixture. -

Avogadro's Concept of Equivalents for Teaching Cation Exchange Capacity
Avogadro’sconcept of equivalentsfor teachingcation exchange1 capacity Steve2 J. Thien ABSTRACT A thoroughunderstanding of cation exchange will have probably learned their chemistry under capacity, whichrequires mastery of manychemical the aforementioned definition, their students will conceptsin an introductorysoils course,is crucialto fully comprehendingthe nature of soils. Student come with another concept in mind. The role of performancein this area hadbeen especially low on chemistry is so basic to agronomic education that courseobjectives requiring working knowledge of the the resultant information gap needs to be recon- conceptof chemicalequivalents. Four specific dif- ciled. The basic simplicity of using Avogadro’s con- ficulties associatedwith understandingand using cept, plus its ability to organize and clarify many equivalentsare examined.Avogadro’s concept of an equivalentas 6.02 X 1023charges is outlinedas an previously difficult concepts to quantitative chemis- alternative pedagogicalapproach. The advantages of try make the new approach educationally attractive. simplicity and readily apparentstoichiometry are This paper uses cation exchange as the agronomic offered in someexamples. The approachhas sig- format for illuminating the advantages of teaching nificantly increasedstudent performance on related agronomic chemistry based on pedagogical use of courseobjectives. Avogadro’s number. Additionalindex words: Soil chemistry,Pedagogi- cal approach. DIFFICULTIES IN LEARNINGEQUIVALENT CHEMISTRY MEDEOAvogadro, a 19th century Italian Cation exchange is a fundamental chemical con- cept used in understanding soil science. Its im- A physicist, is perhaps best remembered for his portance to comprehending the nature of soils is hypothesis that laid the framework for our under- frequently considered parallel to the impact of standing of molecular weights. In addition to dis- photosynthesis in studying crop science. -

Molecular Mass and Gram-Molecule of a Substance
STOICHIOMETRY ATOMIC MASSS The atomic mass of an element is defined as the average relative mass of an atom of the element as compared to the mass of a carbon atom, which is taken as 12. Therefore, the atomic mass of an element shows the number of times an atom of the element is heavier than 1/12th of the mass of a carbon atom (C12). For example, the atomic mass of an oxygen atom is 16 a.m.u (atomic mass unit) and that of a hydrogen atom is 1.008 a.m.u. Atomic mass unit—abbreviated as a.m.u—is defined as the quantity of mass of an atom equal to 1/12 of the mass of a carbon atom. By convention, the atomic mass of a carbon atom is 12 a.m.u. One mole of carbon signifies 6.023 1023 atoms of carbon, and a mass of 12.000 g. Therefore, the mass of a carbon atom can be calculated as follows: 24 12.00/6.023 1023 g or 1 a.m.u. = = = 1.66 10 G Gram-atomic mass of an element is the atomic mass of the element expressed in grams. It is also known as gram atom. For example, 16 g of oxygen is equal to 1 gram-atom of oxygen or 14 g of nitrogen is equal to 1 gram-atom of nitrogen. Gram-atom can be calculated as shown below: Gram atom = Molecular Mass and Gram-Molecule of a Substance Molecular mass of a compound is defined as the average mass per molecule of natural isotopic composition of the compound.